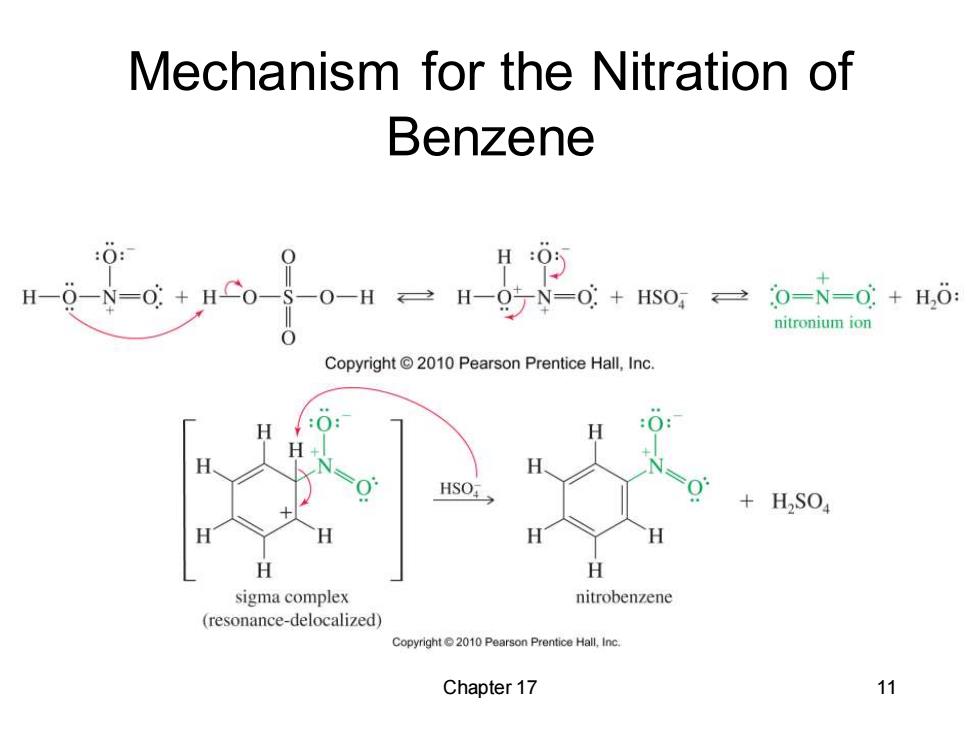
Mechanism for the Nitration of Benzene 0: H0的 8N=0HS00H三H=g7=00 0=N=0+H,0: nitronium ion Copyright 2010 Pearson Prentice Hall,Inc. HSO +H,S04 sigma complex nitrobenzene (resonance-delocalized) Copyright2010 Pearson Prentice Hall,Inc. Chapter 17 11
Chapter 17 11 Mechanism for the Nitration of Benzene
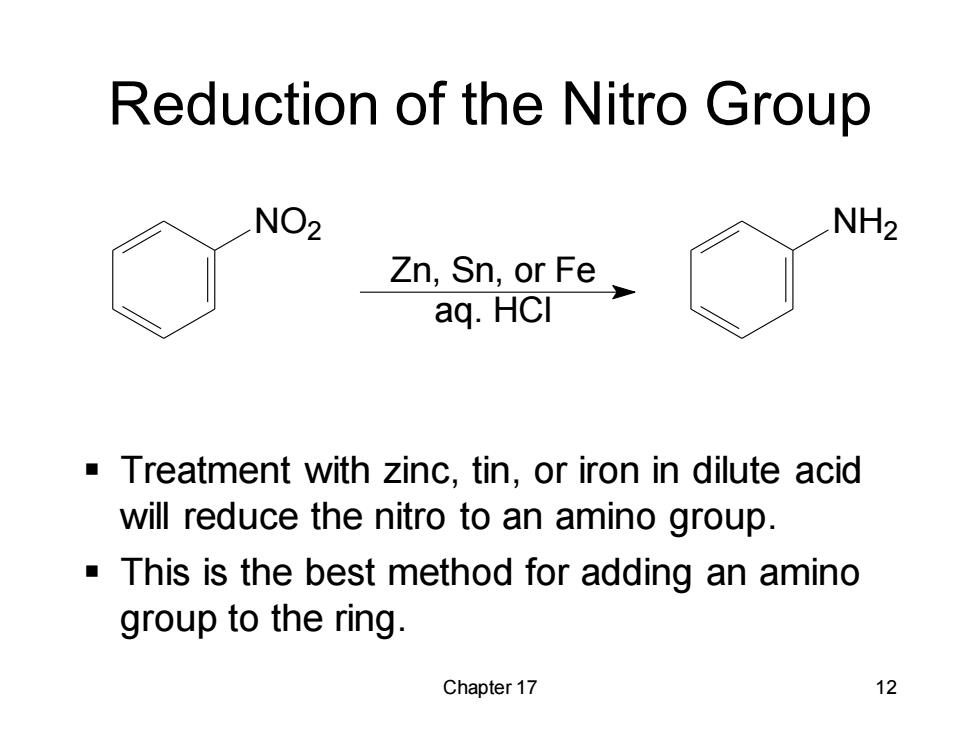
Reduction of the nitro Group NO2 NH2 Zn,Sn,or Fe_ aq.HCI Treatment with zinc,tin,or iron in dilute acid will reduce the nitro to an amino group. This is the best method for adding an amino group to the ring. Chapter 17 12
Chapter 17 12 Reduction of the Nitro Group NO2 Zn, Sn, or Fe aq. HCl NH2 ▪ Treatment with zinc, tin, or iron in dilute acid will reduce the nitro to an amino group. ▪ This is the best method for adding an amino group to the ring
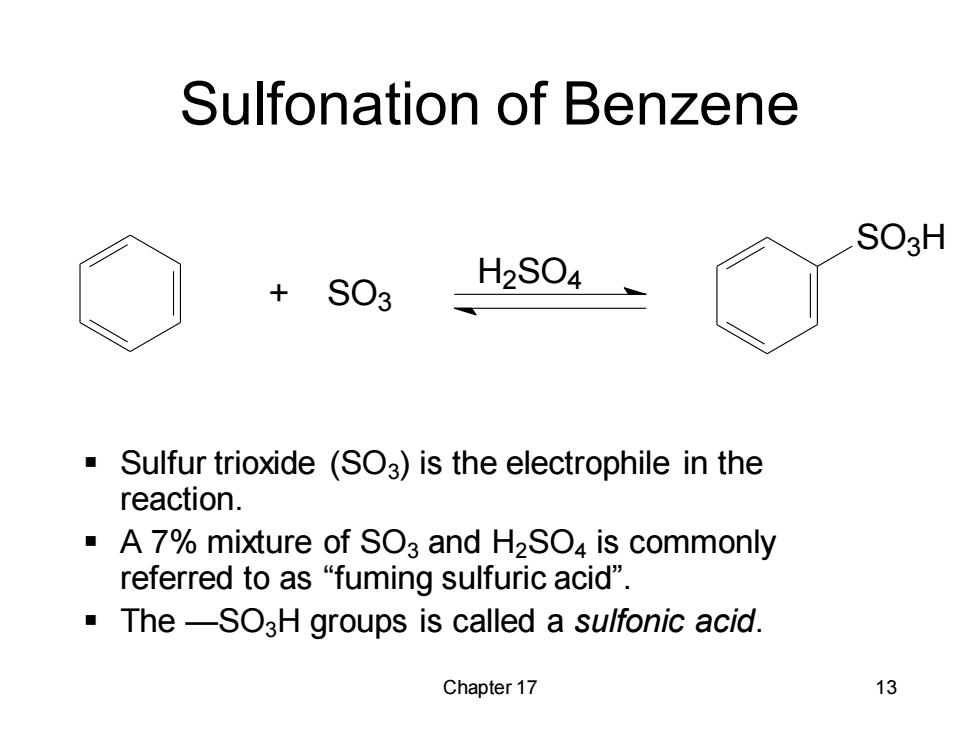
Sulfonation of Benzene SO3H +S03 H2S04 Sulfur trioxide (SO3)is the electrophile in the reaction. A 7%mixture of SO3 and H2SO4 is commonly referred to as "fuming sulfuric acid". The-SO3H groups is called a sulfonic acid. Chapter 17 13
Chapter 17 13 Sulfonation of Benzene ▪ Sulfur trioxide (SO3) is the electrophile in the reaction. ▪ A 7% mixture of SO3 and H2SO4 is commonly referred to as “fuming sulfuric acid”. ▪ The —SO3H groups is called a sulfonic acid. SO3 H + SO3 H2 SO4
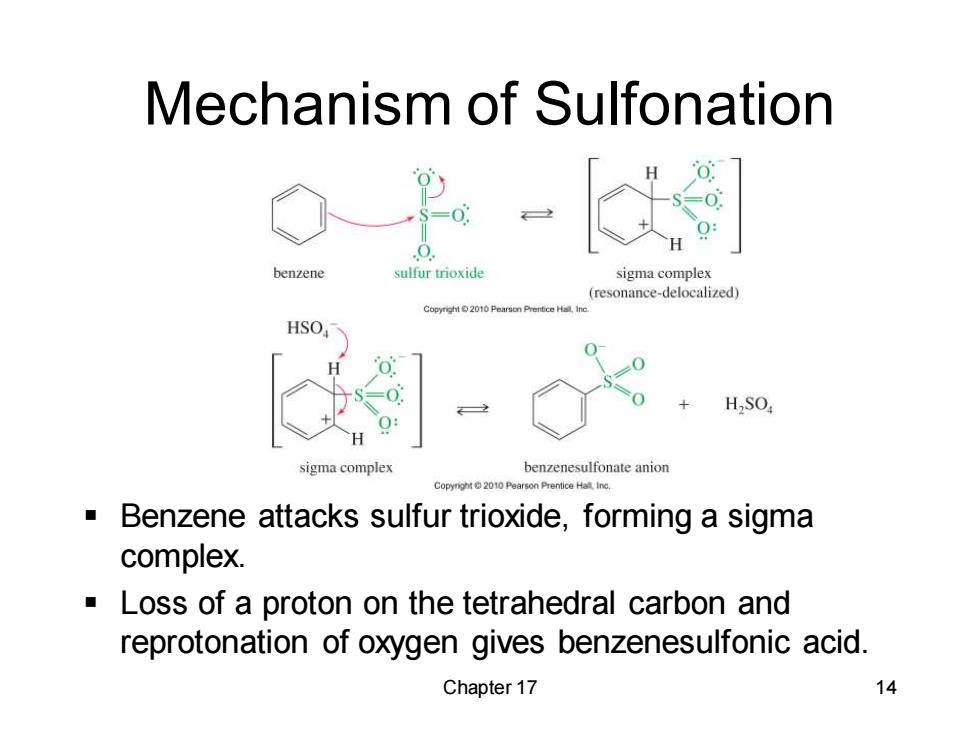
Mechanism of Sulfonation benzene sulfur trioxide sigma complex (resonance-delocalized) HSO HSO sigma complex benzenesulfonate anion C0pyg2010 Benzene attacks sulfur trioxide,forming a sigma complex. Loss of a proton on the tetrahedral carbon and reprotonation of oxygen gives benzenesulfonic acid. Chapter 17 14
Chapter 17 14 Mechanism of Sulfonation ▪ Benzene attacks sulfur trioxide, forming a sigma complex. ▪ Loss of a proton on the tetrahedral carbon and reprotonation of oxygen gives benzenesulfonic acid

Desulfonation Reaction SO3H H H*,heat +H20 H2S04 Sulfonation is reversible. -The sulfonic acid group may be removed from an aromatic ring by heating in dilute sulfuric acid. Chapter 17 15
Chapter 17 15 Desulfonation Reaction ▪ Sulfonation is reversible. ▪ The sulfonic acid group may be removed from an aromatic ring by heating in dilute sulfuric acid. SO3 H H + H2O H + , heat + H2 SO4