
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 24 Amino Acids,Peptides,and Proteins Copyright 2010 Pearson Education,Inc
Chapter 24 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Amino Acids, Peptides, and Proteins
Proteins Biopolymers of a-amino acids. -Amino acids are joined by peptide bond. They serve a variety of functions: ■Structure Enzymes Transport Protection ■Hormones Chapter 24 2
Chapter 24 2 Proteins ▪ Biopolymers of -amino acids. ▪ Amino acids are joined by peptide bond. ▪ They serve a variety of functions: ▪ Structure ▪ Enzymes ▪ Transport ▪ Protection ▪ Hormones
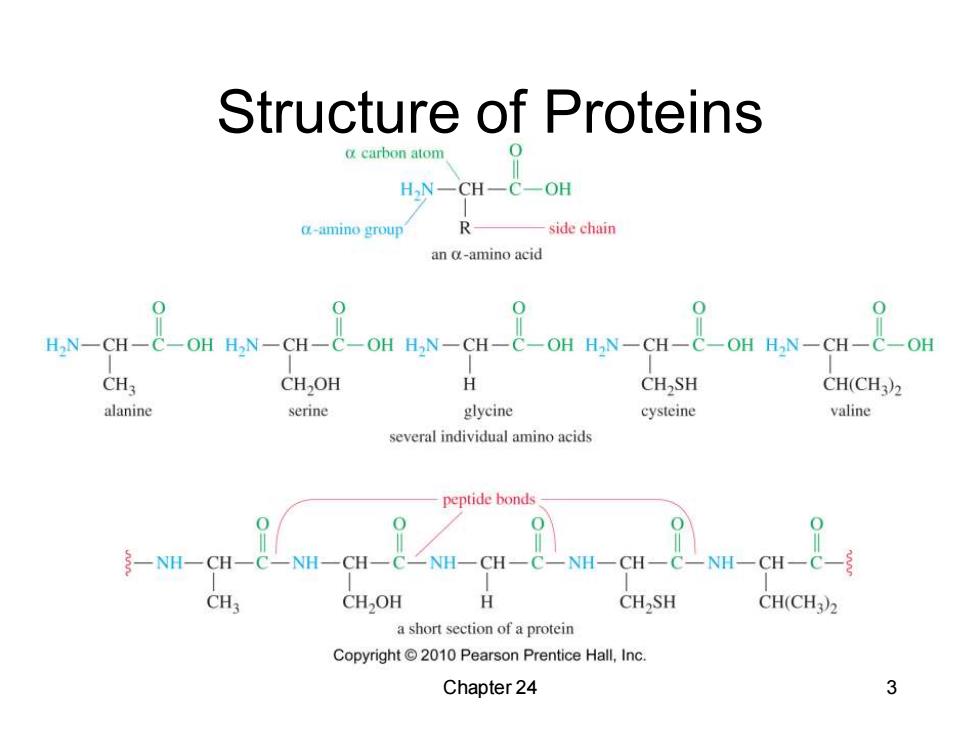
Structure of Proteins 仪carbon atom CH-C- OH a-amino group R —side chain an d-amino acid 0 0 HN-CH-C-OH H2N-CH-C-OH H2N-CH-C- OH HN-CH-C-OH HN-CH-C-OH CH3 CH2OH H CH2SH CH(CH3)2 alanine serine glycine cysteine valine several individual amino acids peptide bonds ;-NH-CH-C-NH-CH- NH一CH NH-CH- NH-CH-C -8 CH3 CH2OH H CH>SH CH(CH32 a short section of a protein Copyright 2010 Pearson Prentice Hall,Inc. Chapter 24 3
Chapter 24 3 Structure of Proteins
Amino Acids -NH2 on the carbon next to -COOH. Glycine,NH2-CH2-COOH,is simplest. -With-R side chain,molecule is chiral. -Most natural amino acids are L-amino acids,related to L-(-)-glyceraldehyde. Direction of optical rotation,(+)or(-), must be determined experimentally. Chapter 24
Chapter 24 4 Amino Acids ▪ —NH2 on the carbon next to —COOH. ▪ Glycine, NH2—CH2—COOH, is simplest. ▪ With —R side chain, molecule is chiral. ▪ Most natural amino acids are L-amino acids, related to L-(-)-glyceraldehyde. ▪ Direction of optical rotation, (+) or (-), must be determined experimentally
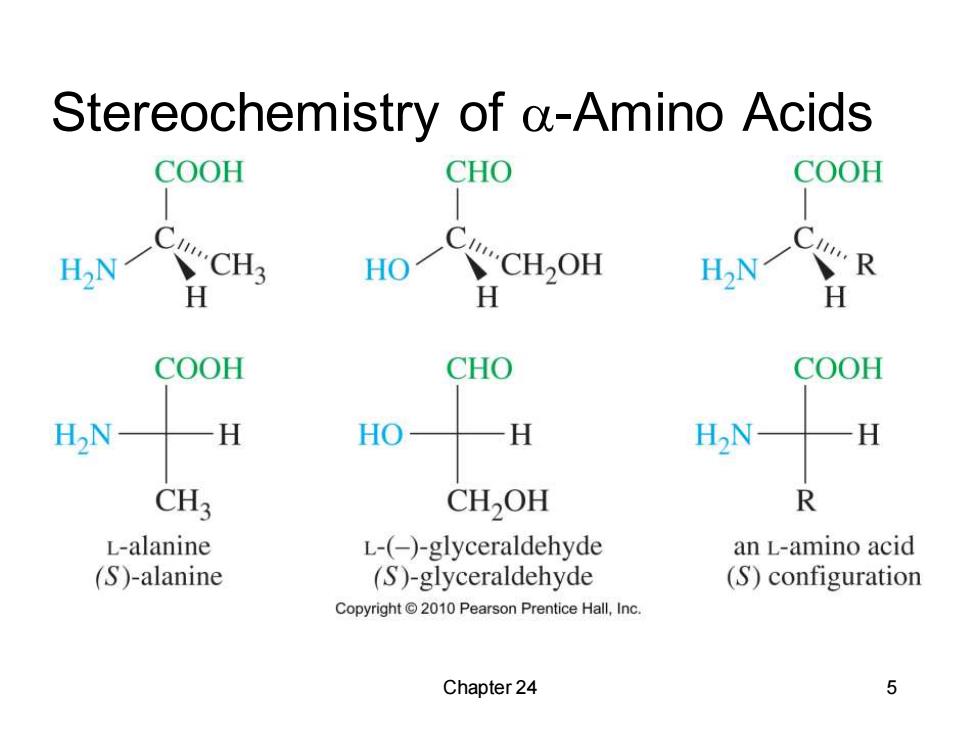
Stereochemistry of a-Amino Acids COOH CHO COOH H H H COOH CHO COOH H2N H HO H H2N- H CH3 CH2OH R L-alanine L-(-)-glyceraldehyde an L-amino acid (S)-alanine (S)-glyceraldehyde (S)configuration Copyright2010 Pearson Prentice Hall,Inc. Chapter 24 5
Chapter 24 5 Stereochemistry of -Amino Acids