
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 16 Lecture Aromatic Compounds 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Chapter 16 1 Chapter 16 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Aromatic Compounds © 2013 Pearson Education, Inc
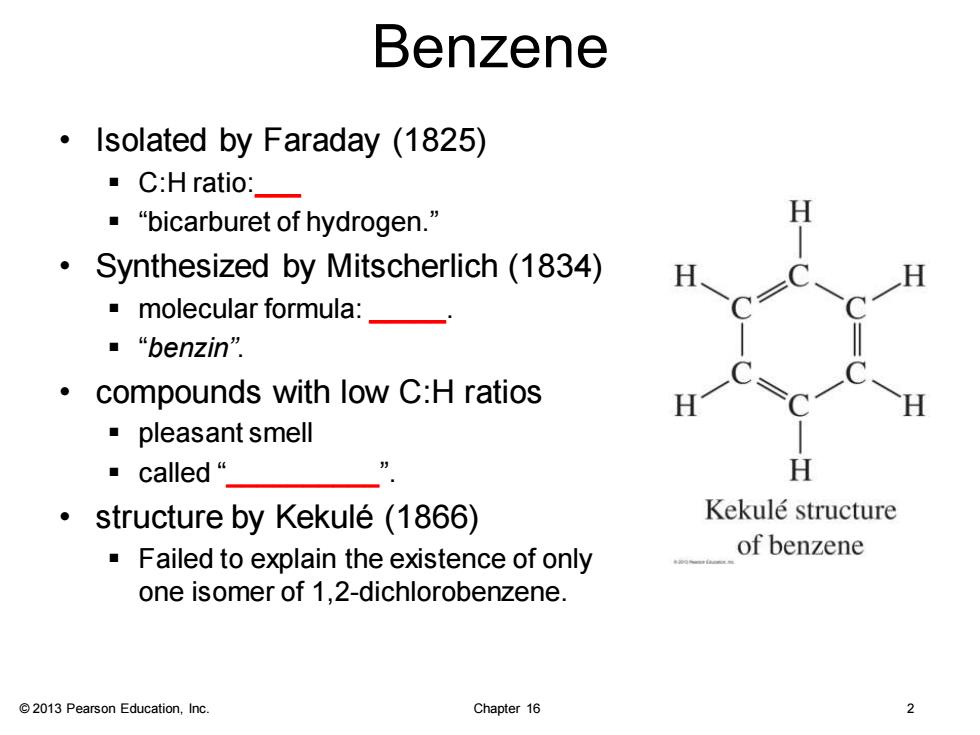
Benzene Isolated by Faraday (1825) ·C:H ratio:- ·“bicarburet of hydrogen.” Synthesized by Mitscherlich(1834) 。molecular formula:_— ■“benzin” compounds with low C:H ratios ·pleasant smell ·called“_ H structure by Kekule (1866) Kekule structure Failed to explain the existence of only of benzene one isomer of 1,2-dichlorobenzene. 2013 Pearson Education,Inc. Chapter 16 2
© 2013 Pearson Education, Inc. Chapter 16 2 Benzene • Isolated by Faraday (1825) ▪ C:H ratio:___ ▪ “bicarburet of hydrogen.” • Synthesized by Mitscherlich (1834) ▪ molecular formula: _____. ▪ “benzin”. • compounds with low C:H ratios ▪ pleasant smell ▪ called “__________”. • structure by Kekulé (1866) ▪ Failed to explain the existence of only one isomer of 1,2-dichlorobenzene
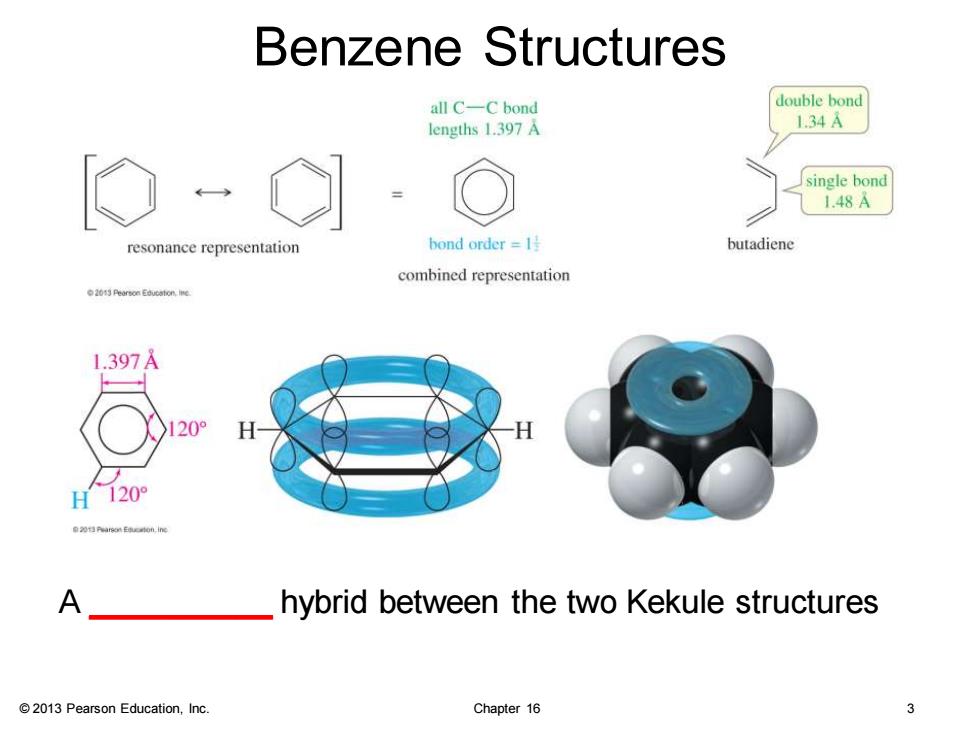
Benzene Structures all C-C bond double bond lengths 1.397A 134A single bond 1.48A resonance representation bond order =1 butadiene combined representation o20特earsonEdo 1.397A H120° A hybrid between the two Kekule structures 2013 Pearson Education,Inc. Chapter 16 3
© 2013 Pearson Education, Inc. Chapter 16 3 Benzene Structures A __________ hybrid between the two Kekule structures
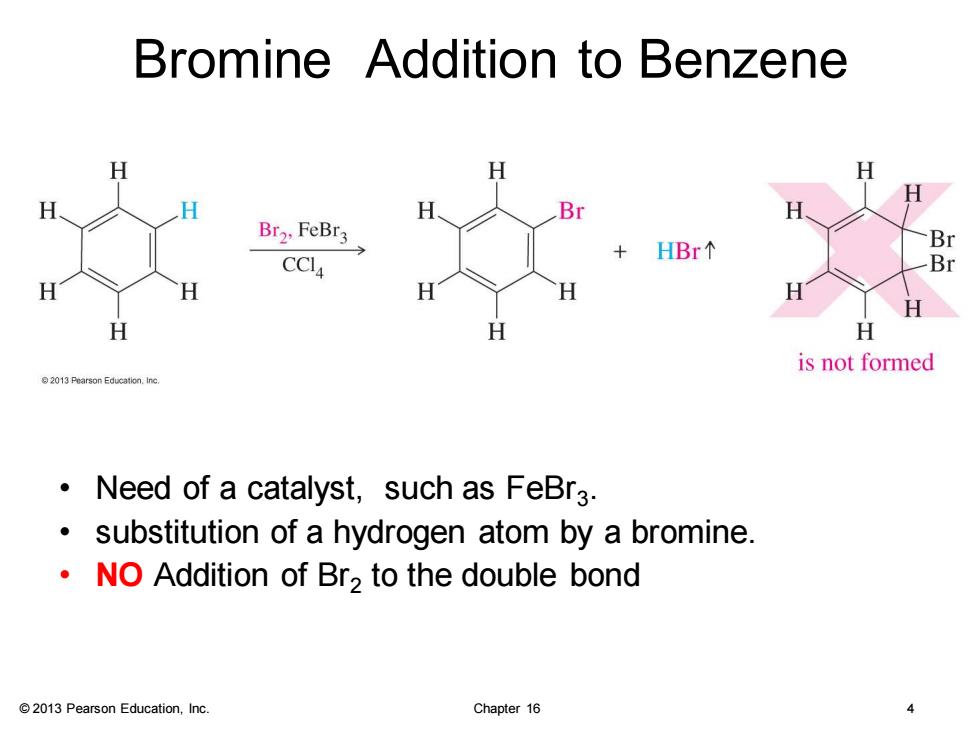
Bromine Addition to Benzene H H H H Br Br2,FeBr3 Br HBr个 CCI4 Br H H H H H H H is not formed 2013 Pearson Education.Inc. Need of a catalyst,such as FeBr3. substitution of a hydrogen atom by a bromine. NO Addition of Br2 to the double bond 2013 Pearson Education,Inc. Chapter 16 4
© 2013 Pearson Education, Inc. Chapter 16 4 Bromine Addition to Benzene • Need of a catalyst, such as FeBr3 . • substitution of a hydrogen atom by a bromine. • NO Addition of Br2 to the double bond
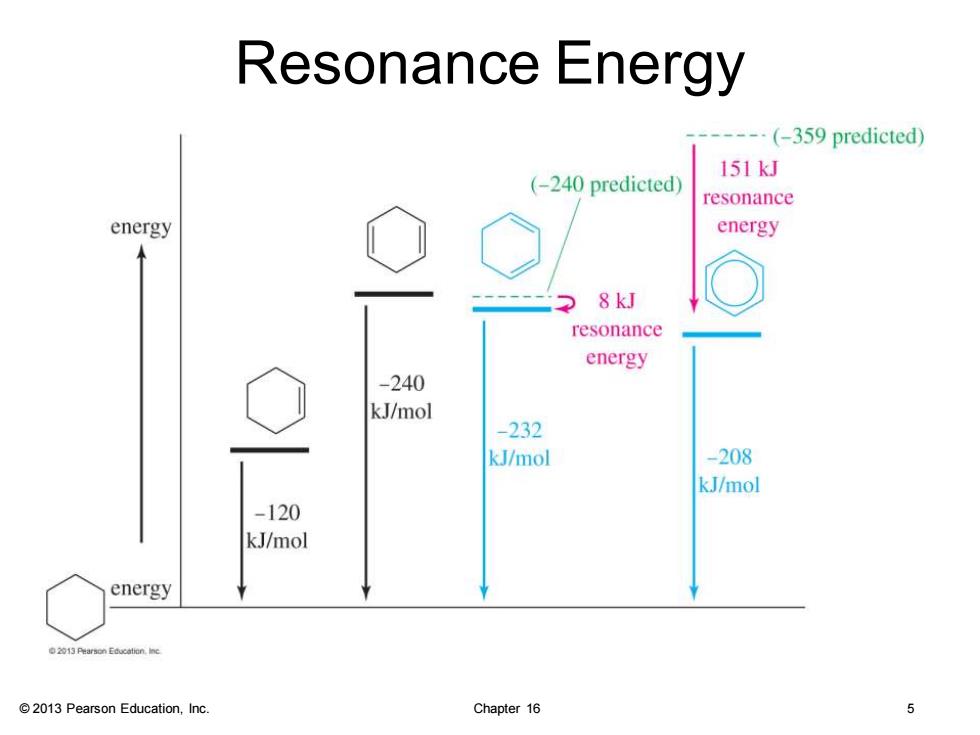
Resonance Energy ---(-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy 2013 Pearson Education,Inc. Chapter 16 5
© 2013 Pearson Education, Inc. Chapter 16 5 Resonance Energy