
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 3 Lecture Structure and Stereochemistry of Alkanes G.WADE,JR Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc ALWAYS LEARNING PEARSON
© 2013 Pearson Education, Inc. Structure and Stereochemistry of Alkanes © 2013 Pearson Education, Inc. Chapter 3 Lecture Rizalia Klausmeyer Baylor University Waco, TX Organic Chemistry, 8 th Edition L. G. Wade, Jr
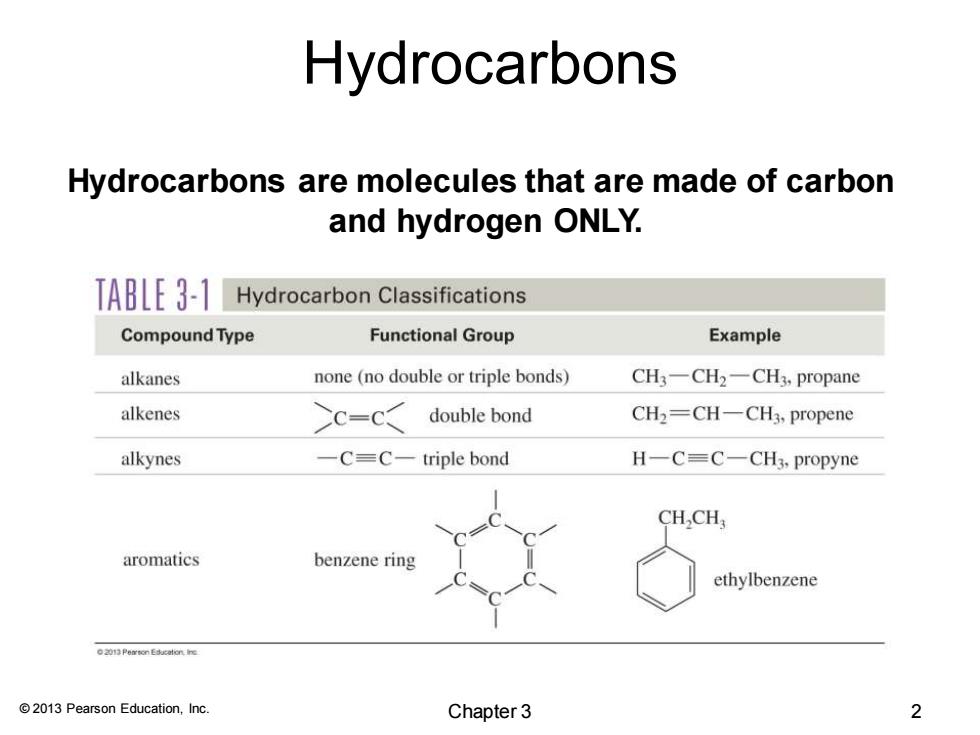
Hydrocarbons Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. TABLE 3-1 Hydrocarbon Classifications Compound Type Functional Group Example alkanes none (no double or triple bonds) CH3-CH2一CH,propane alkenes C=C double bond CH2=CH一CH3,propene alkynes -C=C-triple bond H-C=C-CH3,propyne CH,CH; aromatics benzene ring ethylbenzene 201 Peon Esucationre 2013 Pearson Education,Inc. Chapter 3 2
© 2013 Pearson Education, Inc. Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. Hydrocarbons Chapter 3 2
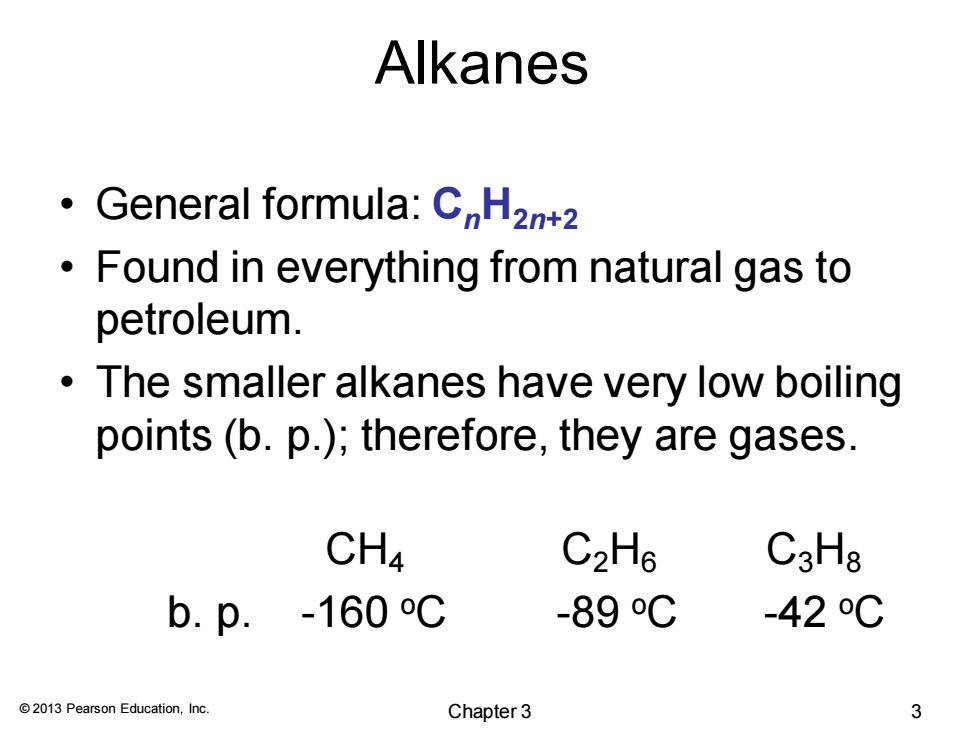
Alkanes General formula:CnHzn+2 Found in everything from natural gas to petroleum. The smaller alkanes have very low boiling points (b.p.);therefore,they are gases. CH4 C2H6 C3Ha b.p.-160℃ -89oC -420C 2013 Pearson Education,Inc. Chapter 3 3
© 2013 Pearson Education, Inc. Alkanes • General formula: CnH2n+2 • Found in everything from natural gas to petroleum. • The smaller alkanes have very low boiling points (b. p.); therefore, they are gases. CH4 C2H6 C3H8 b. p. -160 oC -89 oC -42 oC Chapter 3 3
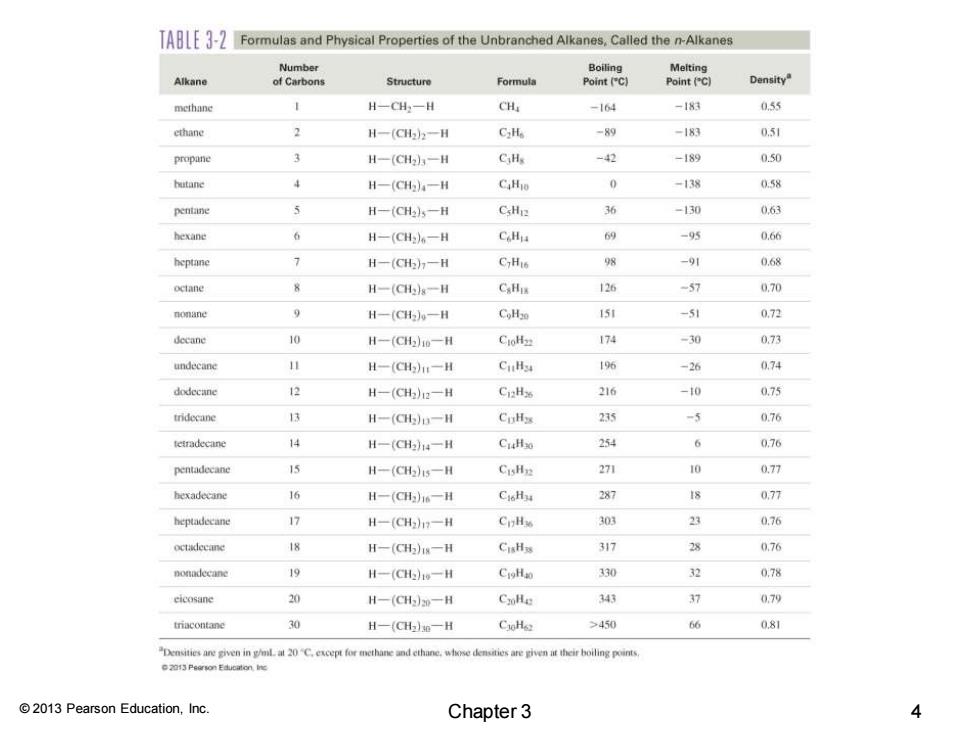
A-Formulas and Physical Properties of the Unbranched Alkanes,Called the n-Alkanes Number Alkane of Carbons Structure Formula Density methane H-CH一H CHa -164 -183 0.55 ethane H-(C52-H C2He -89 -183 051 propane 3 H一(CH3-H C,Hy -42 -189 0.50 hutane 4 H一(CH).-H CaHio -13器 0.58 pentane H-(CH25一H CsHe 安 -130 0.63 hexane H一(CH6一H CHi 多 -95 0.66 heptane H-一(CHn一H CyHis 98 068 octane H一(aHs一H CaHu -7 0,70 monane 9 H一(CHw一H CoHzo 150 -5列 0.72 dccane 10 H-(CHo一H CioHz 174 -30 073 undecane H-(aH11一H C✉ 196 -26 074 dodecane H-(CH2)2一H Ci2H 216 -10 075 tridecane H一(aH一H CuHs 235 -5 0.76 teimadecane 4 H一(C14一H CuaHso 254 0.76 pentadecane H-(aH)s一用 CisHp 271 10 0.77 hexadecane 16 H一(Ghw一H CioHi 287 国 0,77 heptadecane 公 H一(CH1一H CnHs 303 0,76 octadeeane 体 H一(CH5)1g一H CIHs 317 2 0.76 nonadecane H一(CHe一H CioHa 330 32 0,78 cicosane 20 H-(CH)m一H CxHe 34 37 079 H一(CHe一H CxHe >450 66 081 Detvin20C.excepfo mthanedthwhse demsreven hr bolingn 2013 Pearson Education,Inc. Chapter 3 4
© 2013 Pearson Education, Inc. Chapter 3 4
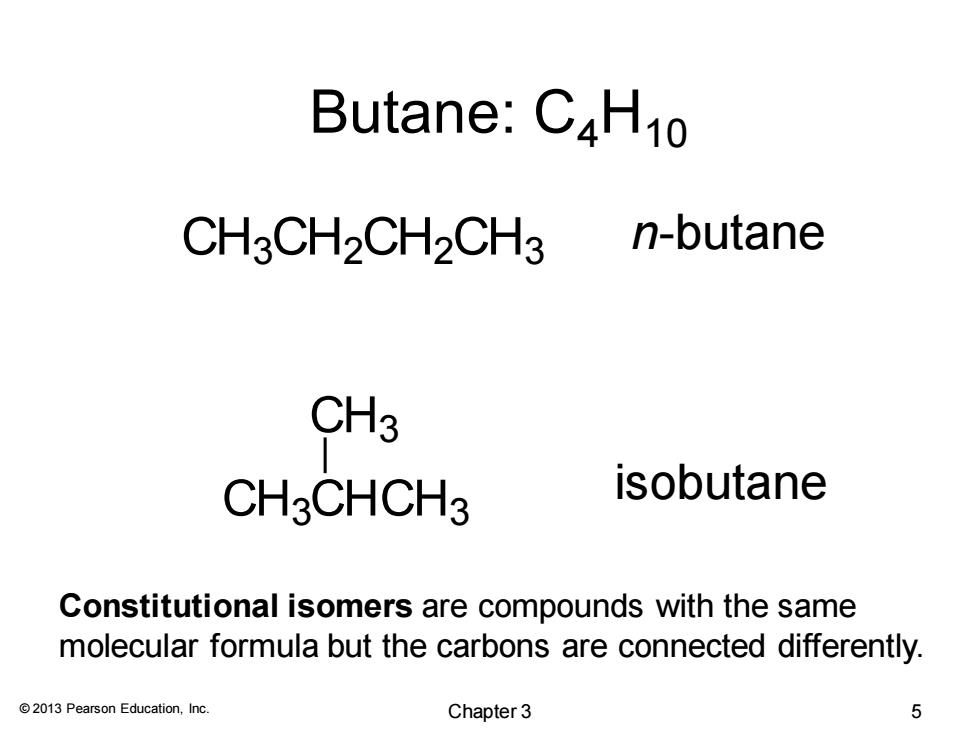
Butane:C4H10 CH3CH2CH2CH3 n-butane CH3 CH3CHCH3 isobutane Constitutional isomers are compounds with the same molecular formula but the carbons are connected differently. 2013 Pearson Education,Inc. Chapter 3 5
© 2013 Pearson Education, Inc. Butane: C4H10 Constitutional isomers are compounds with the same molecular formula but the carbons are connected differently. CH3CH2CH2CH3 CH3CHCH3 CH3 n-butane isobutane Chapter 3 5