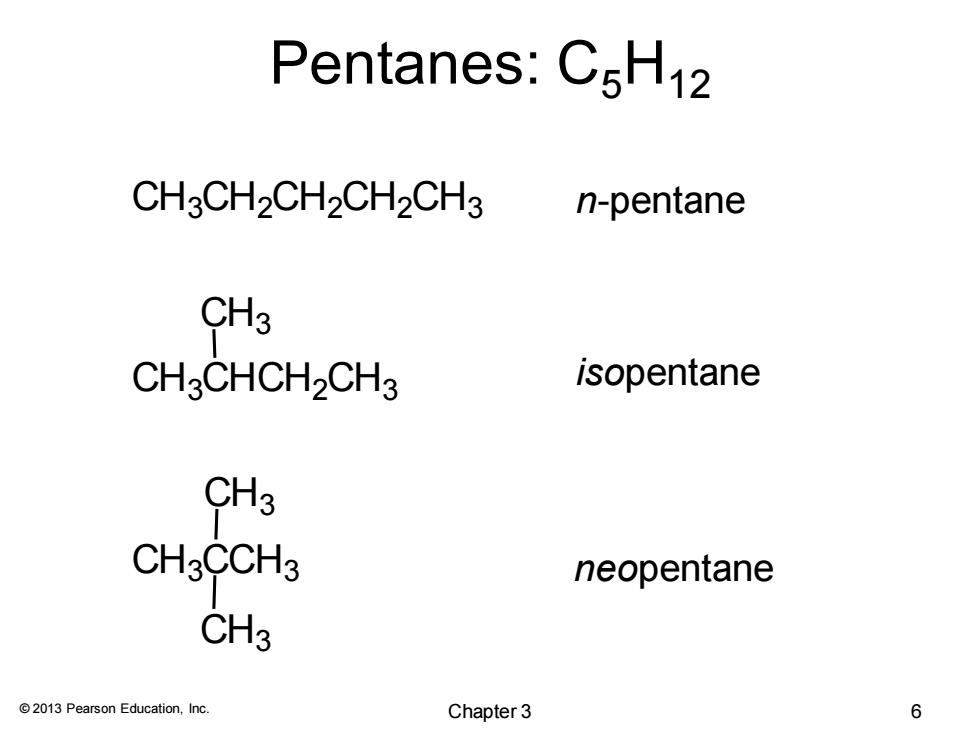
Pentanes:C5H12 CH3CH2CH2CH2CH3 n-pentane CH3 CHCHCH2CH3 isopentane CH3 CH3CCH3 neopentane CH3 2013 Pearson Education,Inc. Chapter 3 6
© 2013 Pearson Education, Inc. Pentanes: C5H12 n-pentane isopentane neopentane CH3CH2CH2CH2CH3 CH3CHCH2CH3 CH3CCH3 CH3 CH3 CH3 Chapter 3 6
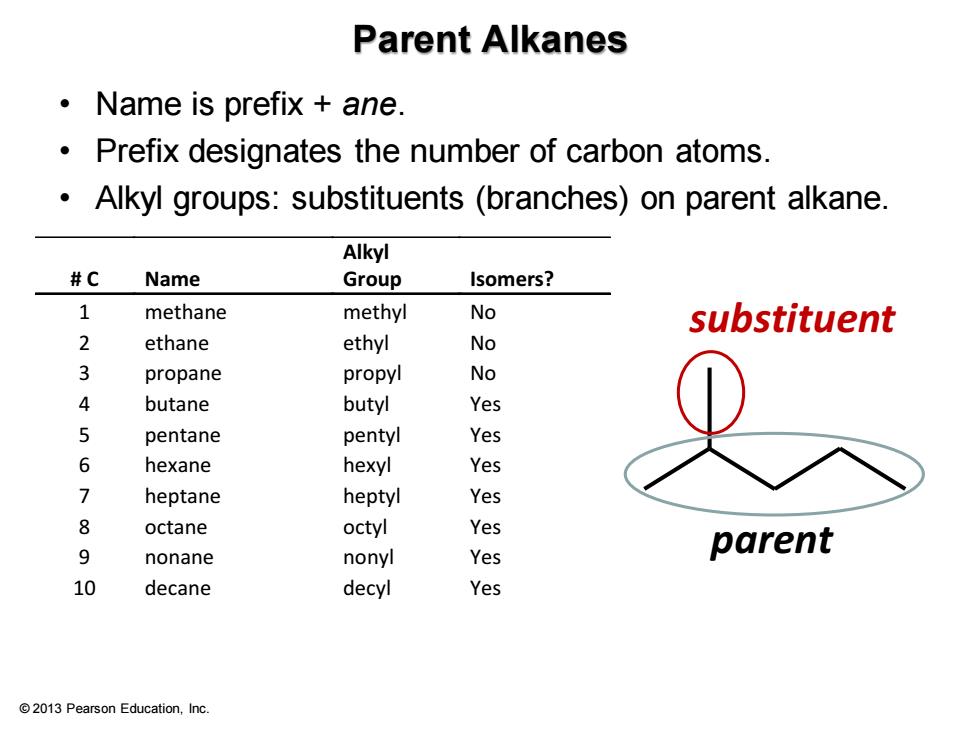
Parent Alkanes ·Name is prefix+ane. Prefix designates the number of carbon atoms. Alkyl groups:substituents (branches)on parent alkane. Alkyl #C Name Group Isomers? 1 methane methyl No substituent 2 ethane ethyl No propane propyl No 4 butane butyl Yes 5 pentane pentyl Yes 6 hexane hexyl Yes 7 heptane heptyl Yes 8 octane octyl Yes 9 nonane nonyl Yes parent 10 decane decyl Yes 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Parent Alkanes • Name is prefix + ane. • Prefix designates the number of carbon atoms. • Alkyl groups: substituents (branches) on parent alkane. # C Name Alkyl Group Isomers? 1 methane methyl No 2 ethane ethyl No 3 propane propyl No 4 butane butyl Yes 5 pentane pentyl Yes 6 hexane hexyl Yes 7 heptane heptyl Yes 8 octane octyl Yes 9 nonane nonyl Yes 10 decane decyl Yes parent substituent
IUPAC or Systematic Names International Union of Pure and Applied Chemistry. Standard method used internationally to name compounds. Uses the longest chain of carbons as the main chain. ● Common names kept:methane,ethane, propane,and butane. 2013 Pearson Education,Inc. Chapter3 8
© 2013 Pearson Education, Inc. IUPAC or Systematic Names • International Union of Pure and Applied Chemistry. • Standard method used internationally to name compounds. • Uses the longest chain of carbons as the main chain. • Common names kept: methane, ethane, propane, and butane. Chapter 3 8

IUPAC Rules Rule 1:Find the longest continuous chain of carbon atoms,and use the name of this chain as the base name of the compound. Rule 2:Number the longest chain,beginning with the end of the chain nearest a substituent. Rule 3:Name the groups attached to the longest chain as alkyl groups.Give the location of each alkyl group by the number of the main-chain carbon atom to which it is attached. Write the alkyl groups in alphabetical order regardless of their position on the chain. 2013 Pearson Education,Inc. Chapter 3 9
© 2013 Pearson Education, Inc. IUPAC Rules • Rule 1: Find the longest continuous chain of carbon atoms, and use the name of this chain as the base name of the compound. • Rule 2: Number the longest chain, beginning with the end of the chain nearest a substituent. • Rule 3: Name the groups attached to the longest chain as alkyl groups. Give the location of each alkyl group by the number of the main-chain carbon atom to which it is attached. • Write the alkyl groups in alphabetical order regardless of their position on the chain. Chapter 3 9
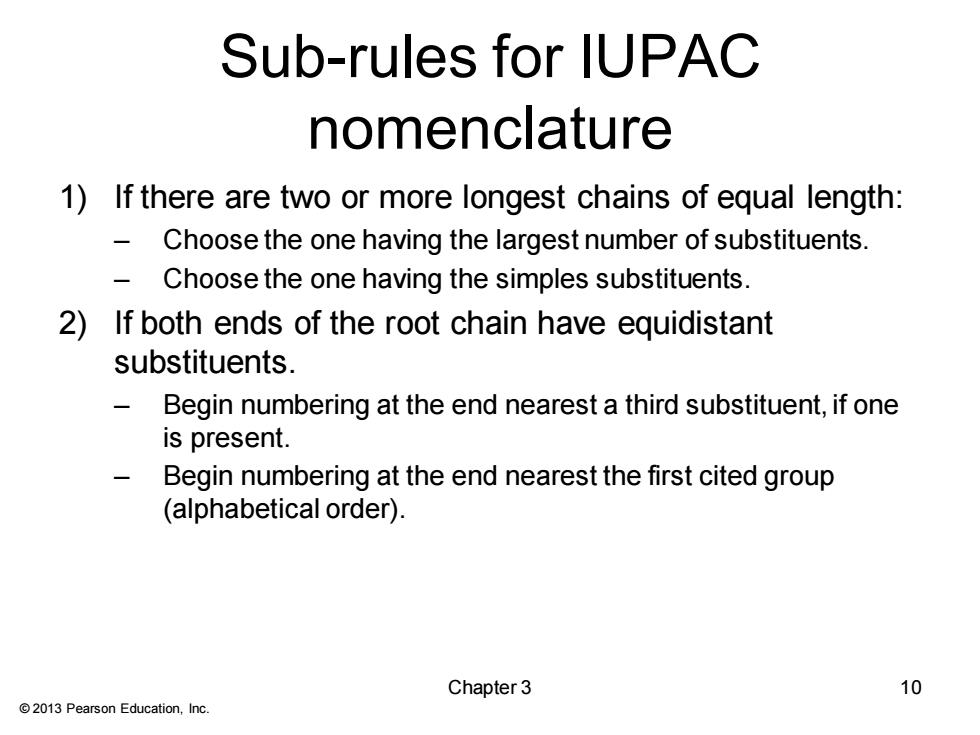
Sub-rules for IUPAC nomenclature 1)If there are two or more longest chains of equal length: Choose the one having the largest number of substituents. Choose the one having the simples substituents. 2)If both ends of the root chain have equidistant substituents. Begin numbering at the end nearest a third substituent,if one is present. Begin numbering at the end nearest the first cited group (alphabetical order). Chapter 3 10 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Sub-rules for IUPAC nomenclature 1) If there are two or more longest chains of equal length: – Choose the one having the largest number of substituents. – Choose the one having the simples substituents. 2) If both ends of the root chain have equidistant substituents. – Begin numbering at the end nearest a third substituent, if one is present. – Begin numbering at the end nearest the first cited group (alphabetical order). Chapter 3 10