
REACTION MECHANISM and SYNTHESIS REVIEW
REACTION MECHANISM and SYNTHESIS REVIEW
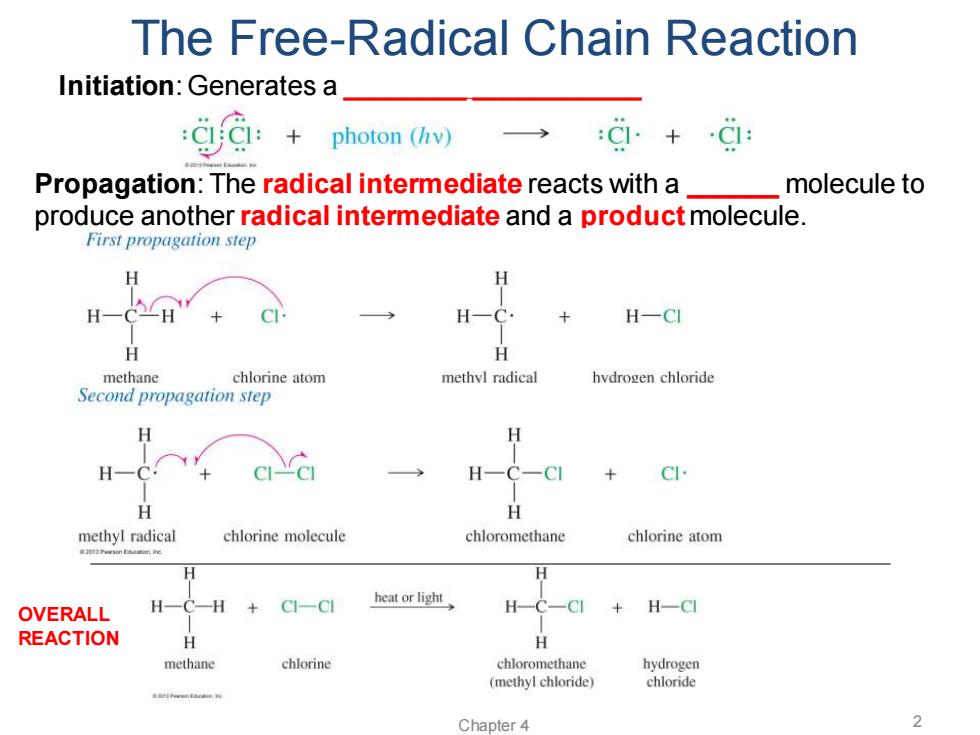
The Free-Radical Chain Reaction Initiation:Generates a CIC:+ photon(hv)→ Propagation:The radical intermediate reacts with a molecule to produce another radical intermediate and a product molecule. First propagation step H H-CI H methane chlorine atom methvl radical hvdrogen chloride Second propagation step H H H H + H H methyl radical chlorine molecule chloromethane chlorine atom OVERALL H- H CI-CI heat or light H H-CI REACTION H methane chlorine chloromethane hydrogen (methyl chloride) chloride smieenlacam he Chapter 4 2
Chapter 4 2 The Free-Radical Chain Reaction Initiation: Generates a ________ ___________ Propagation: The radical intermediate reacts with a ______ molecule to produce another radical intermediate and a product molecule. OVERALL REACTION
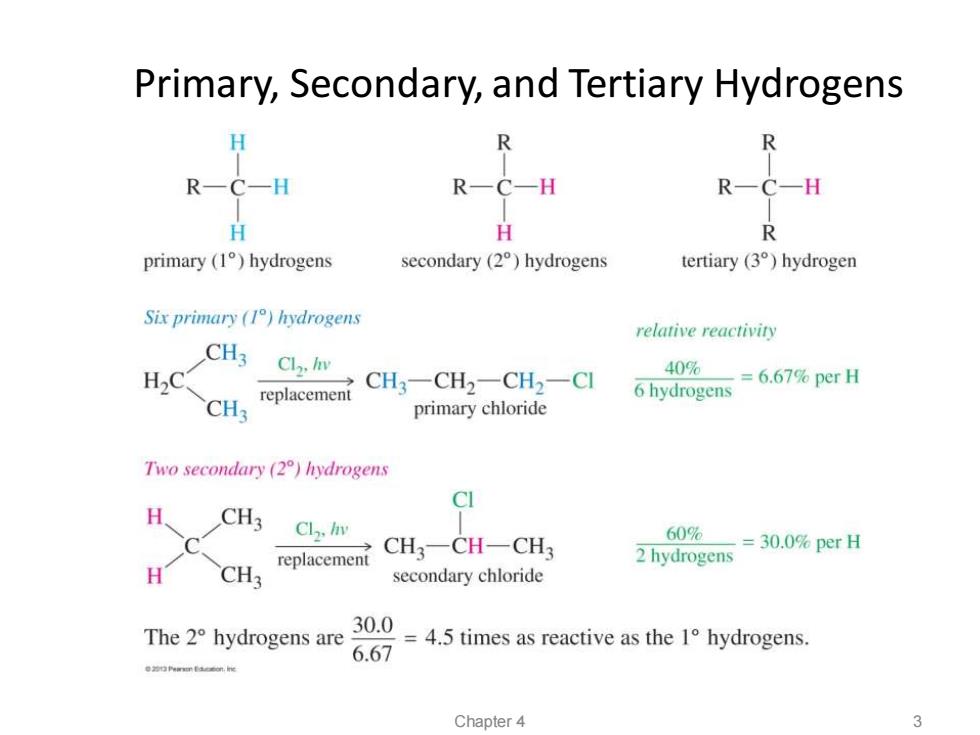
Primary,Secondary,and Tertiary Hydrogens H R RCH R一C-H R一C-H H H R primary (1)hydrogens secondary(2)hydrogens tertiary (3)hydrogen Six primary (I)hydrogens relative reactiviry CH3 Cl,lnv 40% H2C CH3一CH2一CH2-CI 6 hydrogens =6.67%perH CH3 replacement primary chloride Two secondary (2)hydrogens CI H CH3 Cl./n 60% CH3一CH一CH3 -=30.0%per日 replacement 2 hydrogens H CH3 secondary chloride The2°hydrogens are 30.0 6.67 =4.5 times as reactive as the 1 hydrogens. Chapter 4 3
Primary, Secondary, and Tertiary Hydrogens Chapter 4 3
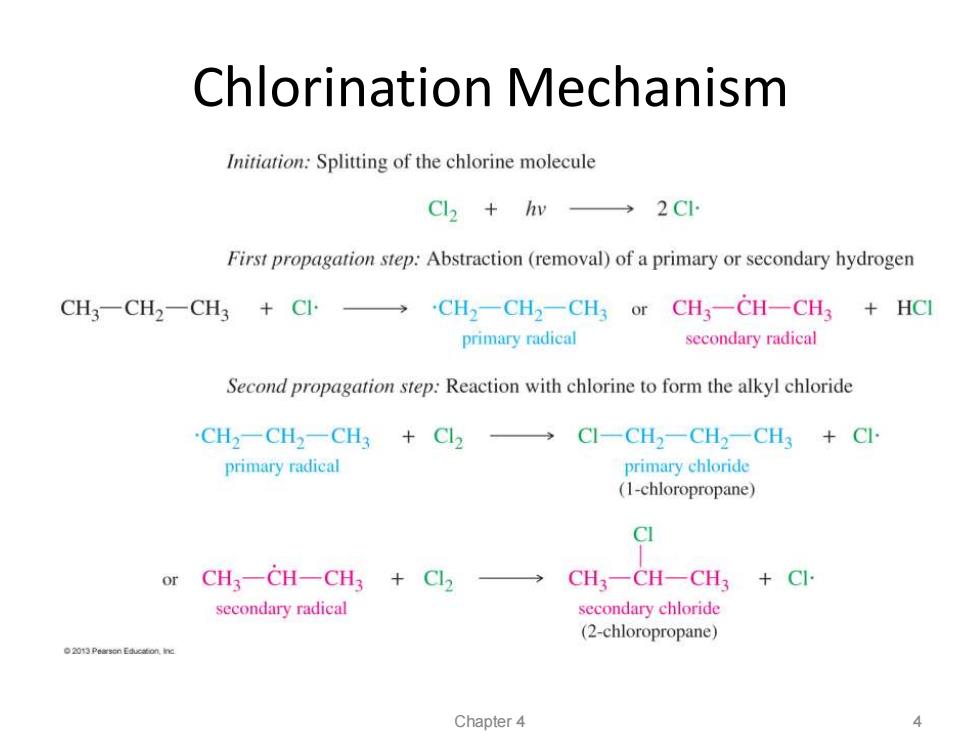
Chlorination Mechanism Initiation:Splitting of the chlorine molecule Cl2+hv→2C First propagation step:Abstraction(removal)of a primary or secondary hydrogen CH3-CH2-CH3+CI→·CH2一CH2-CH3 or CH3-CH-CH3+HC primary radical secondary radical Second propagation step:Reaction with chlorine to form the alkyl chloride CH2一CH2-CH3+C2 →C1-CH2-CH2-CH3+C primary radical primary chloride (1-chloropropane) CI or CH3-CH-CH3 Cl2 → CH3-CH-CH3 Cl. secondary radical secondary chloride (2-chloropropane) 2013 Pearsen Education ine Chapter 4
Chlorination Mechanism Chapter 4 4
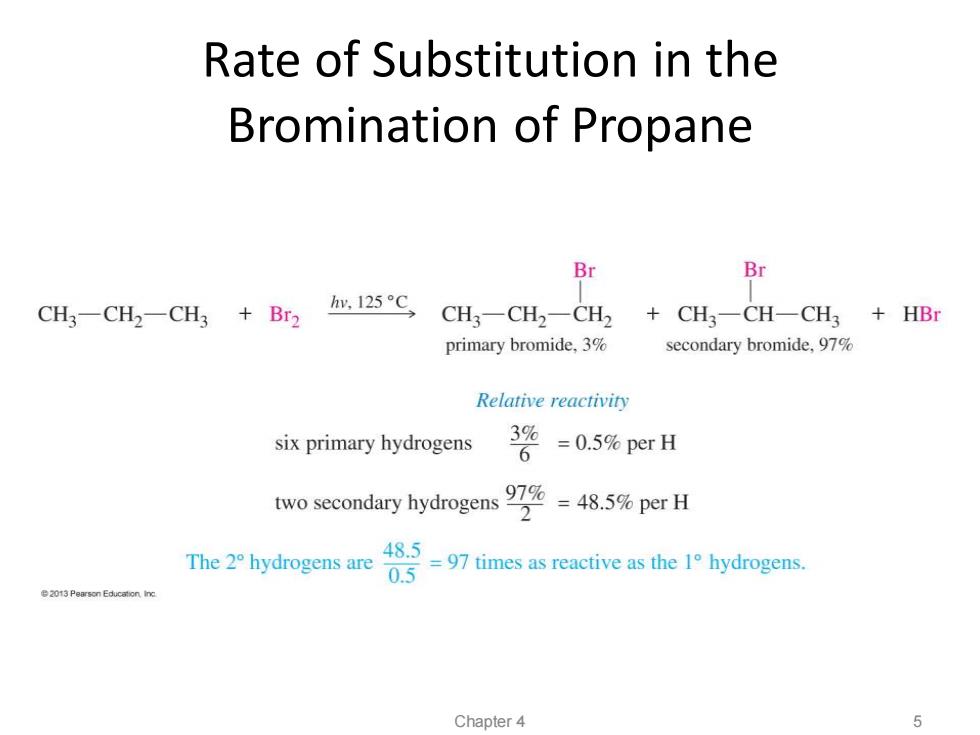
Rate of Substitution in the Bromination of Propane Br Br CH3-CH2-CH3 +Br2 hy.125C CH3-CH2一CH2 +CH3-CH-CH3 +HBr primary bromide,3% secondary bromide,97% Relative reactivity six primary hydrogens 3% 6 =0.5%per H two secondary hydrogens48.%per H 2 The 2 hydrogens are 48.5 0.5 =97 times as reactive as the 1 hydrogens. Chapter 4 5
Rate of Substitution in the Bromination of Propane Chapter 4 5