
CNGNGE JOHN MCMURRY CHAPTER 11 Structure Determination: Nuclear Magnetic Resonance T H IR D E DI Spectroscopy Organic Chemistry with Biological Applications
CHAPTER 11 Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Introduction Nuclear Magnetic Resonance (NMR)Spectroscopy is the most valuable spectroscopic technique available to laboratory organic chemists Taken together,mass spectrometry,IR,UV and NMR make it possible to determine the structures of even very complex molecules Mass spectrometry molecular formula Infrared Spectroscopy functional groups UV Spectroscopy extent of conjugation NMR Spectroscopy map of carbon-hydrogen framework
• Nuclear Magnetic Resonance (NMR) Spectroscopy is the most valuable spectroscopic technique available to laboratory organic chemists • Taken together, mass spectrometry, IR, UV and NMR make it possible to determine the structures of even very complex molecules Mass spectrometry - molecular formula Infrared Spectroscopy - functional groups UV Spectroscopy - extent of conjugation NMR Spectroscopy - map of carbon-hydrogen framework Introduction

11-1 Nuclear Magnetic Resonance Spectroscopy Many atomic nuclei behave as if they spin on an axis of rotation -Nuclei are positively charged These spinning nuclei generate tiny magnetic fields Tiny magnets interact with an external magnetic field,denoted Bo Proton (1H)and carbon (13C)are the most important nuclear spins to organic chemists
Many atomic nuclei behave as if they spin on an axis of rotation ▪ Nuclei are positively charged ▪ These spinning nuclei generate tiny magnetic fields ▪ Tiny magnets interact with an external magnetic field, denoted B0 ▪ Proton (1H) and carbon (13C) are the most important nuclear spins to organic chemists 11-1 Nuclear Magnetic Resonance Spectroscopy
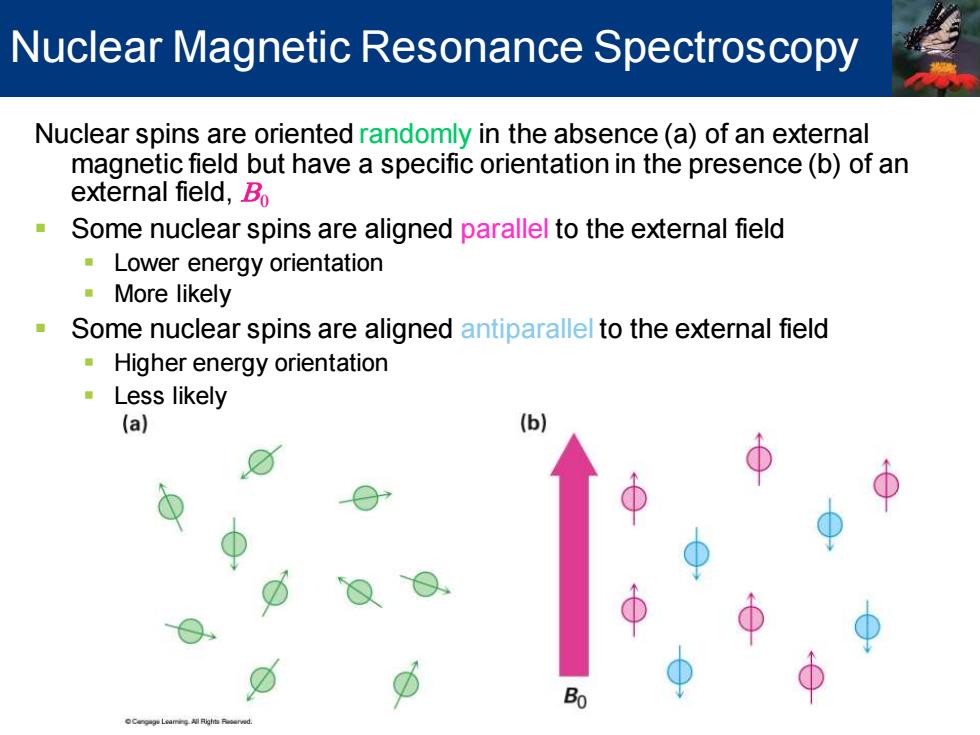
Nuclear Magnetic Resonance Spectroscopy Nuclear spins are oriented randomly in the absence (a)of an external magnetic field but have a specific orientation in the presence(b)of an external field,Bo n Some nuclear spins are aligned parallel to the external field Lower energy orientation More likely Some nuclear spins are aligned antiparallel to the external field Higher energy orientation Less likely (a) (b) 0
Nuclear spins are oriented randomly in the absence (a) of an external magnetic field but have a specific orientation in the presence (b) of an external field, B0 ▪ Some nuclear spins are aligned parallel to the external field ▪ Lower energy orientation ▪ More likely ▪ Some nuclear spins are aligned antiparallel to the external field ▪ Higher energy orientation ▪ Less likely Nuclear Magnetic Resonance Spectroscopy
Nuclear Magnetic Resonance Spectroscopy When nuclei that are aligned parallel with an external magnetic field are irradiated with the proper frequency of electromagnetic radiation the energy is absorbed and the nuclei“spin-flips”to the higher-energy antiparallel alignment Nuclei that undergo "spin-flips"in response to applied radiation are said to be in resonance with the applied radiation -nuclear magnetic resonance Frequency necessary for resonance depends on strength of external field and the identity of the nuclei
When nuclei that are aligned parallel with an external magnetic field are irradiated with the proper frequency of electromagnetic radiation the energy is absorbed and the nuclei “spin-flips” to the higher-energy antiparallel alignment ▪ Nuclei that undergo “spin-flips” in response to applied radiation are said to be in resonance with the applied radiation - nuclear magnetic resonance ▪ Frequency necessary for resonance depends on strength of external field and the identity of the nuclei Nuclear Magnetic Resonance Spectroscopy