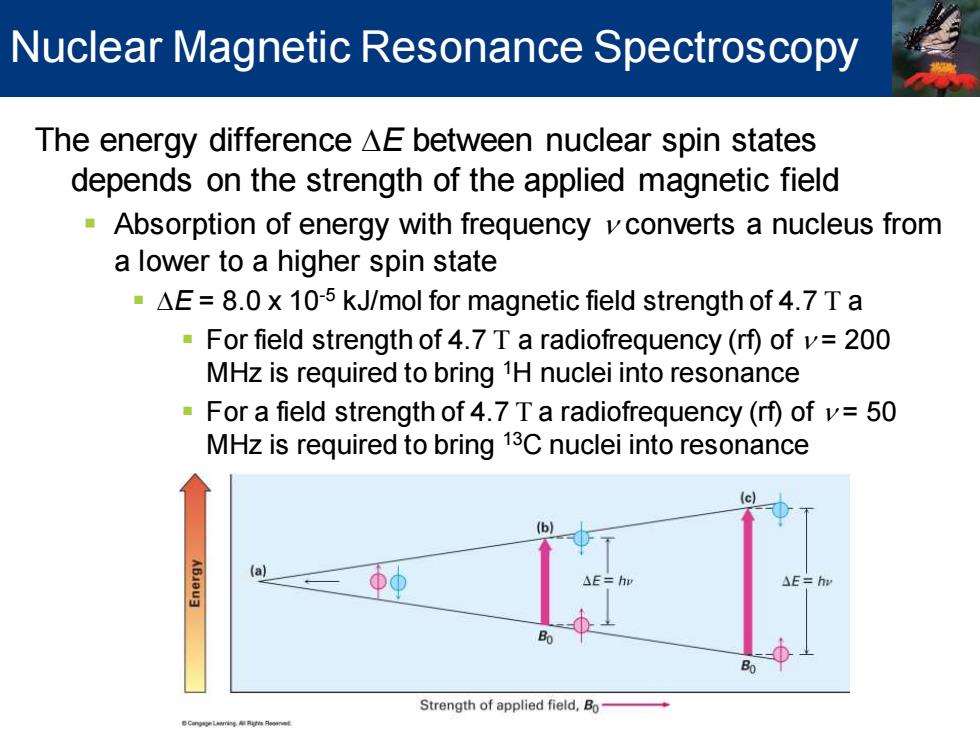
Nuclear Magnetic Resonance Spectroscopy The energy difference AE between nuclear spin states depends on the strength of the applied magnetic field Absorption of energy with frequency vconverts a nucleus from a lower to a higher spin state -AE=8.0 x 10-5 kJ/mol for magnetic field strength of 4.7 T a For field strength of 4.7 T a radiofrequency (rf)of v=200 MHz is required to bring 1H nuclei into resonance For a field strength of 4.7 T a radiofrequency(rf)ofv=50 MHz is required to bring 13C nuclei into resonance (c) (b) Strength of applied field,Bo
The energy difference DE between nuclear spin states depends on the strength of the applied magnetic field ▪ Absorption of energy with frequency n converts a nucleus from a lower to a higher spin state ▪ DE = 8.0 x 10-5 kJ/mol for magnetic field strength of 4.7 T a ▪ For field strength of 4.7 T a radiofrequency (rf) of n = 200 MHz is required to bring 1H nuclei into resonance ▪ For a field strength of 4.7 T a radiofrequency (rf) of n = 50 MHz is required to bring 13C nuclei into resonance Nuclear Magnetic Resonance Spectroscopy
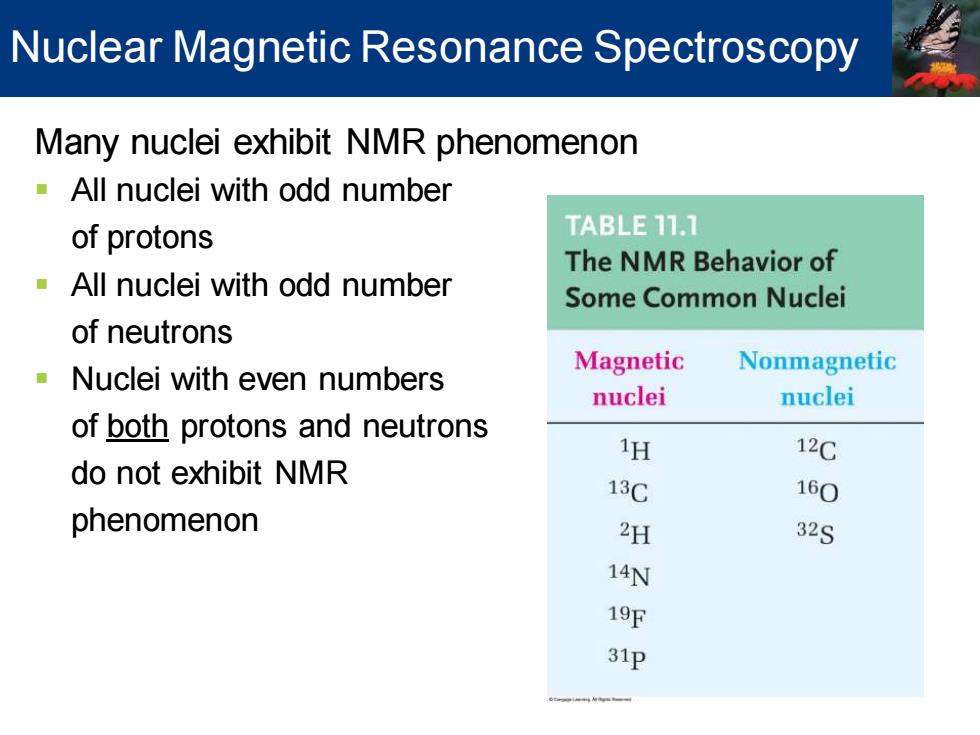
Nuclear Magnetic Resonance Spectroscopy Many nuclei exhibit NMR phenomenon -All nuclei with odd number of protons TABLE 11.1 The NMR Behavior of All nuclei with odd number Some Common Nuclei of neutrons Nuclei with even numbers Magnetic Nonmagnetic nuclei nuclei of both protons and neutrons 1H 12C do not exhibit NMR 13C 160 phenomenon 2H 32S 14N 19℉ 31p
Many nuclei exhibit NMR phenomenon ▪ All nuclei with odd number of protons ▪ All nuclei with odd number of neutrons ▪ Nuclei with even numbers of both protons and neutrons do not exhibit NMR phenomenon Nuclear Magnetic Resonance Spectroscopy
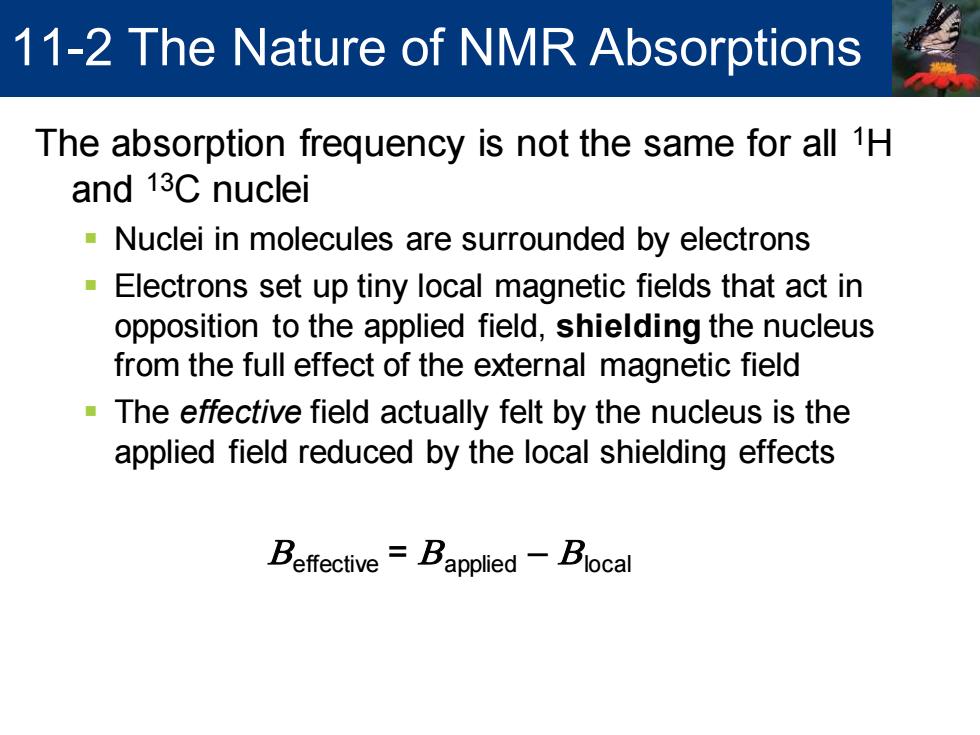
11-2 The Nature of NMR Absorptions The absorption frequency is not the same for all 1H and 13C nuclei Nuclei in molecules are surrounded by electrons Electrons set up tiny local magnetic fields that act in opposition to the applied field,shielding the nucleus from the full effect of the external magnetic field The effective field actually felt by the nucleus is the applied field reduced by the local shielding effects Beffective=Bapplied-Blocal
The absorption frequency is not the same for all 1H and 13C nuclei ▪ Nuclei in molecules are surrounded by electrons ▪ Electrons set up tiny local magnetic fields that act in opposition to the applied field, shielding the nucleus from the full effect of the external magnetic field ▪ The effective field actually felt by the nucleus is the applied field reduced by the local shielding effects Beffective = Bapplied – Blocal 11-2 The Nature of NMR Absorptions
The Nature of NMR Absorptions The absorption frequency is not the same for all 1H and 13C nuclei Each chemically distinct nucleus in a molecule has a slightly different electronic environment and consequently a different effective field Each chemically distinct 13C or 1H nucleus in a molecule experiences a different effective field and will exhibit a distinct 13C or 1H NMR signal
The absorption frequency is not the same for all 1H and 13C nuclei ▪ Each chemically distinct nucleus in a molecule has a slightly different electronic environment and consequently a different effective field ▪ Each chemically distinct 13C or 1H nucleus in a molecule experiences a different effective field and will exhibit a distinct 13C or 1H NMR signal The Nature of NMR Absorptions
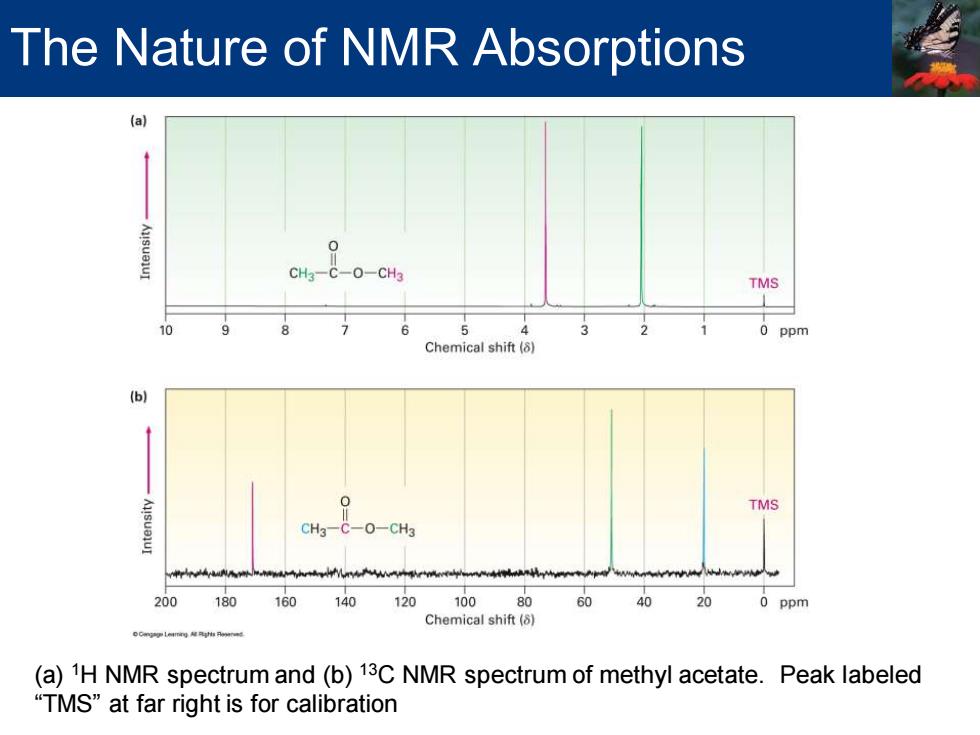
The Nature of NMR Absorptions CH3-C-0-CH3 TMS 6 A 2 0 ppm Chemical shift (8) (b) TMS CH3-C-O-CH3 200180 160 140 12010080 60 40 20 0 ppm Chemical shift(8) (a)1H NMR spectrum and(b)13C NMR spectrum of methyl acetate.Peak labeled “TMS”at far right is for calibration
(a) 1H NMR spectrum and (b) 13C NMR spectrum of methyl acetate. Peak labeled “TMS” at far right is for calibration The Nature of NMR Absorptions