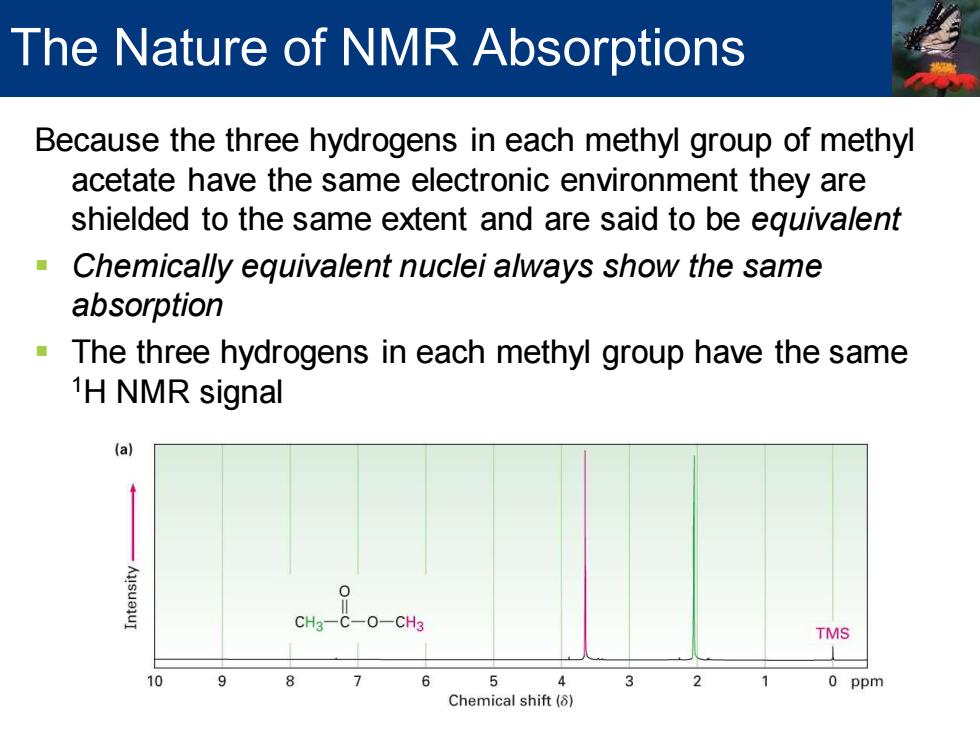
The Nature of NMR Absorptions Because the three hydrogens in each methyl group of methyl acetate have the same electronic environment they are shielded to the same extent and are said to be eguivalent Chemically equivalent nuclei always show the same absorption The three hydrogens in each methyl group have the same 1H NMR signal (a) CH3-C-O-CH3 TMS 10 9 8 5 4 3 2 0 ppm Chemical shift(⑧)
Because the three hydrogens in each methyl group of methyl acetate have the same electronic environment they are shielded to the same extent and are said to be equivalent ▪ Chemically equivalent nuclei always show the same absorption ▪ The three hydrogens in each methyl group have the same 1H NMR signal The Nature of NMR Absorptions
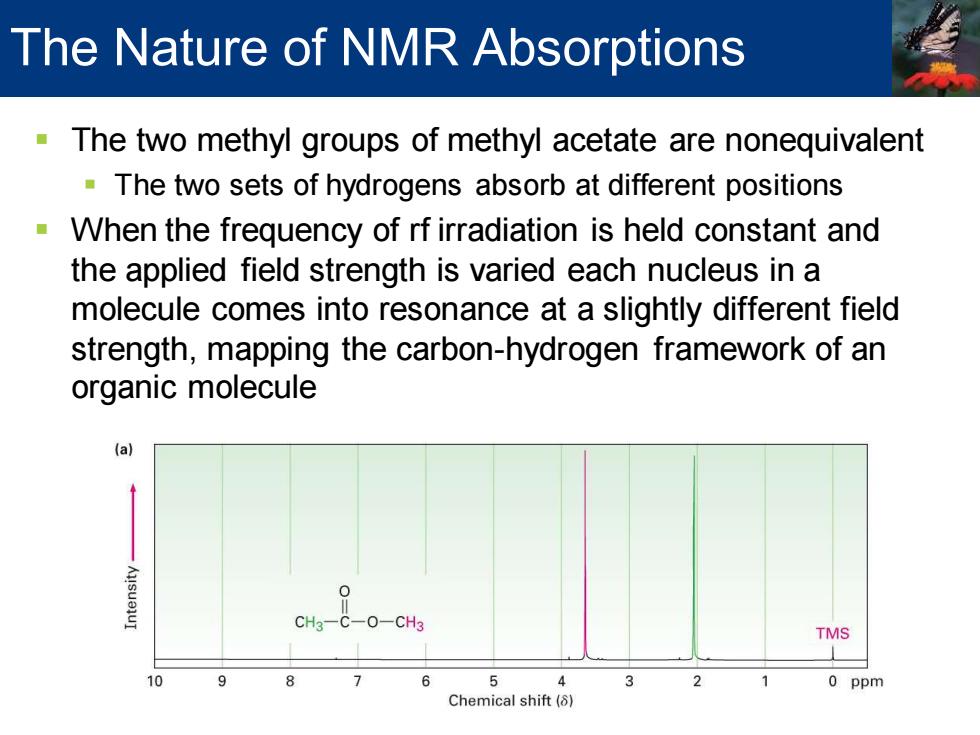
The Nature of NMR Absorptions The two methyl groups of methyl acetate are nonequivalent The two sets of hydrogens absorb at different positions When the frequency of rf irradiation is held constant and the applied field strength is varied each nucleus in a molecule comes into resonance at a slightly different field strength,mapping the carbon-hydrogen framework of an organic molecule (a CH3-C-O-CH3 TMS 10 9 8 7 5 3 0 ppm Chemical shift(8)
▪ The two methyl groups of methyl acetate are nonequivalent ▪ The two sets of hydrogens absorb at different positions ▪ When the frequency of rf irradiation is held constant and the applied field strength is varied each nucleus in a molecule comes into resonance at a slightly different field strength, mapping the carbon-hydrogen framework of an organic molecule The Nature of NMR Absorptions

The Nature of NMR Absorptions The 13C spectrum of methyl acetate shows three peaks,one for each of the three chemically distinct carbon atoms in the molecule (b) TMS AusueluI CH3-C-O-CH3 200 180 160 140 120 100 80 60 40 20 0 ppm Chemical shift(8) Cnpge Leaming Nll Righes Reserwd
The 13C spectrum of methyl acetate shows three peaks, one for each of the three chemically distinct carbon atoms in the molecule The Nature of NMR Absorptions
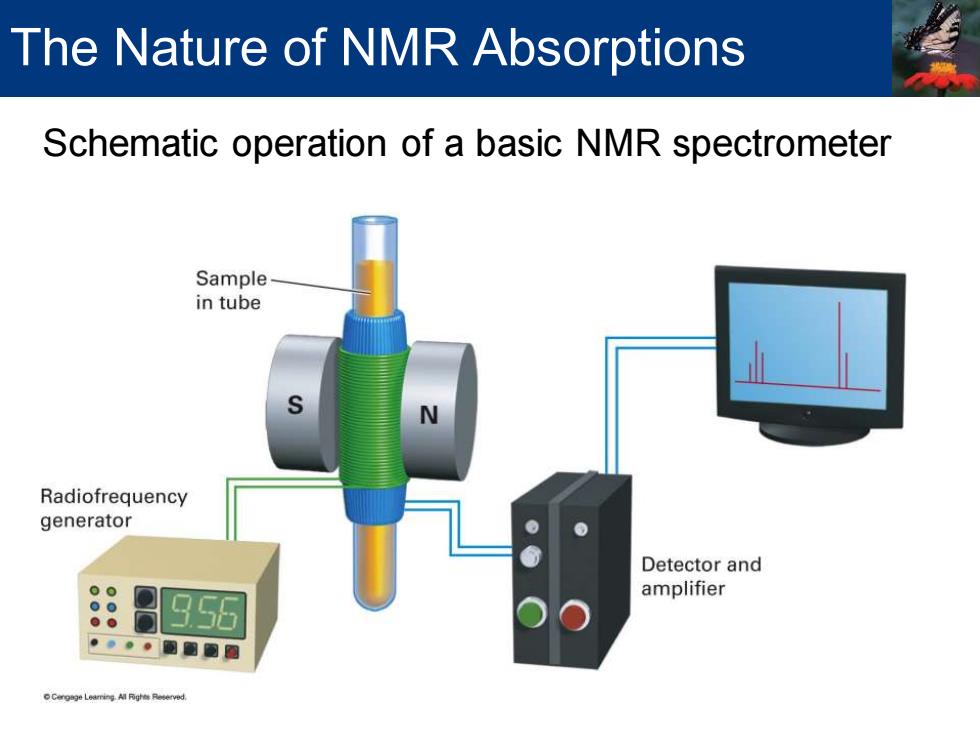
The Nature of NMR Absorptions Schematic operation of a basic NMR spectrometer Sample in tube Radiofrequency generator Detector and amplifier 956 Cergnge Learing All Pighes Reserve时
Schematic operation of a basic NMR spectrometer The Nature of NMR Absorptions

The Nature of NMR Absorptions NMR spectroscopy requires more time(about 10-3 s)compared to IR spectroscopy (about 10-13 s) If two rapidly interconverting species are present in a sample,IR spectroscopy will record spectra for both but the slower NMR spectroscopy will record a "time-averaged"spectrum "Time-averaging"of NMR spectra can be used to measure reaction rates and activation energies of very fast processes H H Eact=45 kJ/mol H 1H NMR:1 peak at 25C 2 peaks at-90C
▪ NMR spectroscopy requires more time (about 10-3 s) compared to IR spectroscopy (about 10-13 s) ▪ If two rapidly interconverting species are present in a sample, IR spectroscopy will record spectra for both but the slower NMR spectroscopy will record a “time-averaged” spectrum ▪ “Time-averaging” of NMR spectra can be used to measure reaction rates and activation energies of very fast processes The Nature of NMR Absorptions