
CNGNGE JOHN MCMURRY CHAPTER 13 Alcohols,Phenols,and Thiols;Ethers and Sulfides EDITION Organic Chemistry with Biological Applications
CHAPTER 13 Alcohols, Phenols, and Thiols; Ethers and Sulfides
Alcohols,Phenols,and Ethers Alcohols,Phenols,and Ethers Organic derivatives of water in which one or both of the water hydrogens is replaced by an organic group:H-O-H versus R-O-H,Ar-O-H,and R-O-R' Thiols and sulfides Corresponding sulfur analogs,R-S-H and R-S-R
Alcohols, Phenols, and Ethers ▪ Organic derivatives of water in which one or both of the water hydrogens is replaced by an organic group: H-O-H versus R-O-H, Ar-O-H, and R-O-R’ Thiols and sulfides ▪ Corresponding sulfur analogs, R-S-H and R-S-R’ Alcohols, Phenols, and Ethers

Alcohols,Phenols,and Ethers The names alcohol and thiol are restricted to compounds that have their -OH or -SH group bonded to a saturated,sp3-hybridized carbon atom Phenols and thiophenols are compounds with their-OH or -SH bonded to an aromatic ring 。 Enols and enethiols are compounds with the-OH or-SH bonded to a vinylic,sp2-hybridized carbon OH OH An alcohol A thiol A phenol A thiophenol An enol An enethiol
The names alcohol and thiol are restricted to compounds that have their –OH or –SH group bonded to a saturated, sp3 -hybridized carbon atom ▪ Phenols and thiophenols are compounds with their –OH or –SH bonded to an aromatic ring ▪ Enols and enethiols are compounds with the –OH or –SH bonded to a vinylic, sp2 -hybridized carbon Alcohols, Phenols, and Ethers
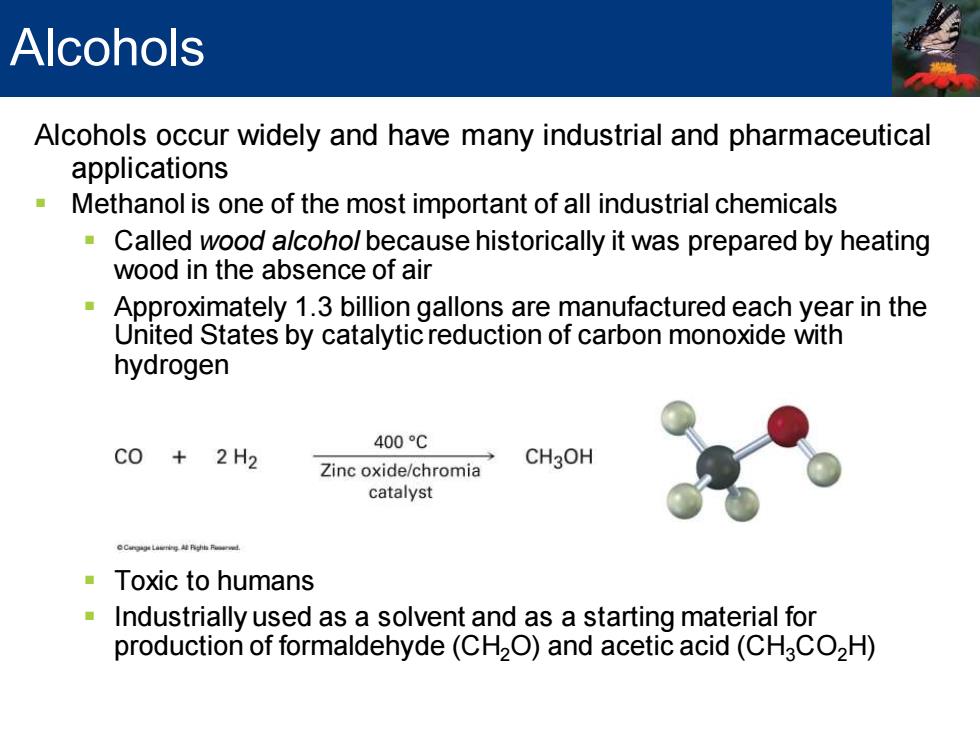
Alcohols Alcohols occur widely and have many industrial and pharmaceutical applications Methanol is one of the most important of all industrial chemicals Called wood alcohol because historically it was prepared by heating wood in the absence of air Approximately 1.3 billion gallons are manufactured each year in the United States by catalytic reduction of carbon monoxide with hydrogen 400℃ C0+2H2 Zinc oxide/chromia CH3OH catalyst Toxic to humans Industrially used as a solvent and as a starting material for production of formaldehyde(CH2O)and acetic acid(CHCO2H)
Alcohols occur widely and have many industrial and pharmaceutical applications ▪ Methanol is one of the most important of all industrial chemicals ▪ Called wood alcohol because historically it was prepared by heating wood in the absence of air ▪ Approximately 1.3 billion gallons are manufactured each year in the United States by catalytic reduction of carbon monoxide with hydrogen ▪ Toxic to humans ▪ Industrially used as a solvent and as a starting material for production of formaldehyde (CH2O) and acetic acid (CH3CO2H) Alcohols
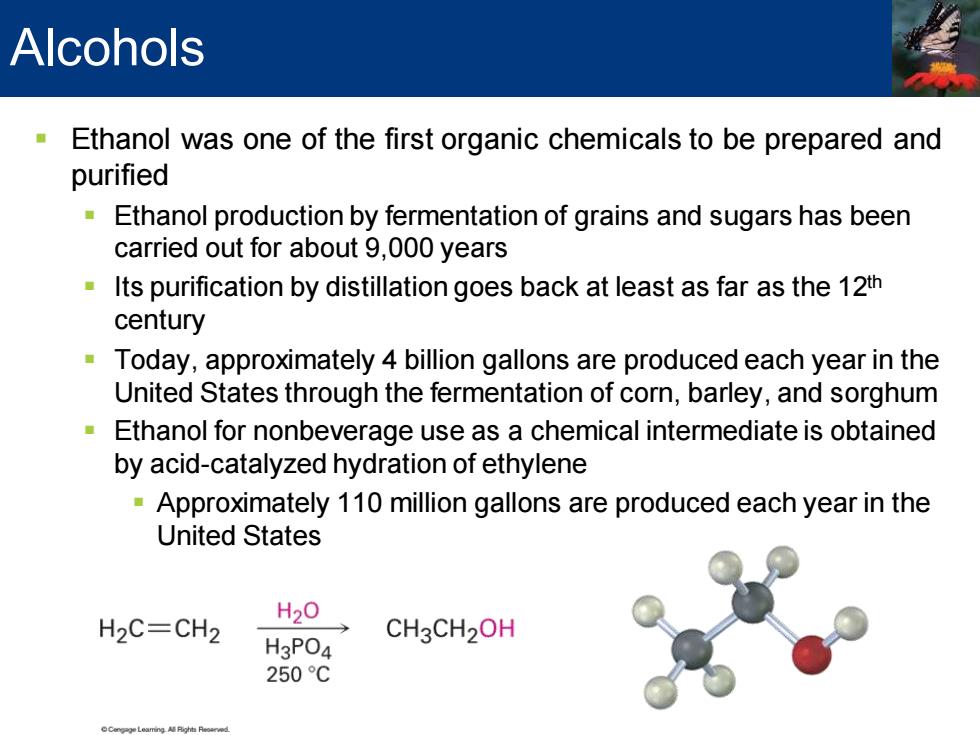
Alcohols Ethanol was one of the first organic chemicals to be prepared and purified Ethanol production by fermentation of grains and sugars has been carried out for about 9,000 years Its purification by distillation goes back at least as far as the 12th century Today,approximately 4 billion gallons are produced each year in the United States through the fermentation of corn,barley,and sorghum Ethanol for nonbeverage use as a chemical intermediate is obtained by acid-catalyzed hydration of ethylene Approximately 110 million gallons are produced each year in the United States H20 H2C=CH2 H3PO4 CH3CH2OH 250C
▪ Ethanol was one of the first organic chemicals to be prepared and purified ▪ Ethanol production by fermentation of grains and sugars has been carried out for about 9,000 years ▪ Its purification by distillation goes back at least as far as the 12th century ▪ Today, approximately 4 billion gallons are produced each year in the United States through the fermentation of corn, barley, and sorghum ▪ Ethanol for nonbeverage use as a chemical intermediate is obtained by acid-catalyzed hydration of ethylene ▪ Approximately 110 million gallons are produced each year in the United States Alcohols