
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 25 Lipids Copyright 2010 Pearson Education,Inc
Chapter 25 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Lipids
Introduction Lipids are compounds that can be extracted from cells by nonpolar organic solvents. Complex lipids are easily hydrolyzed. Long-chain esters called fatty acids. -Simple lipids are not easily hydrolyzed in acid or base. ·Steroids ·Prostaglandins ■Terpenes Chapter 25 2
Chapter 25 2 Introduction ▪ Lipids are compounds that can be extracted from cells by nonpolar organic solvents. ▪ Complex lipids are easily hydrolyzed. ▪ Long-chain esters called fatty acids. ▪ Simple lipids are not easily hydrolyzed in acid or base. ▪ Steroids ▪ Prostaglandins ▪ Terpenes
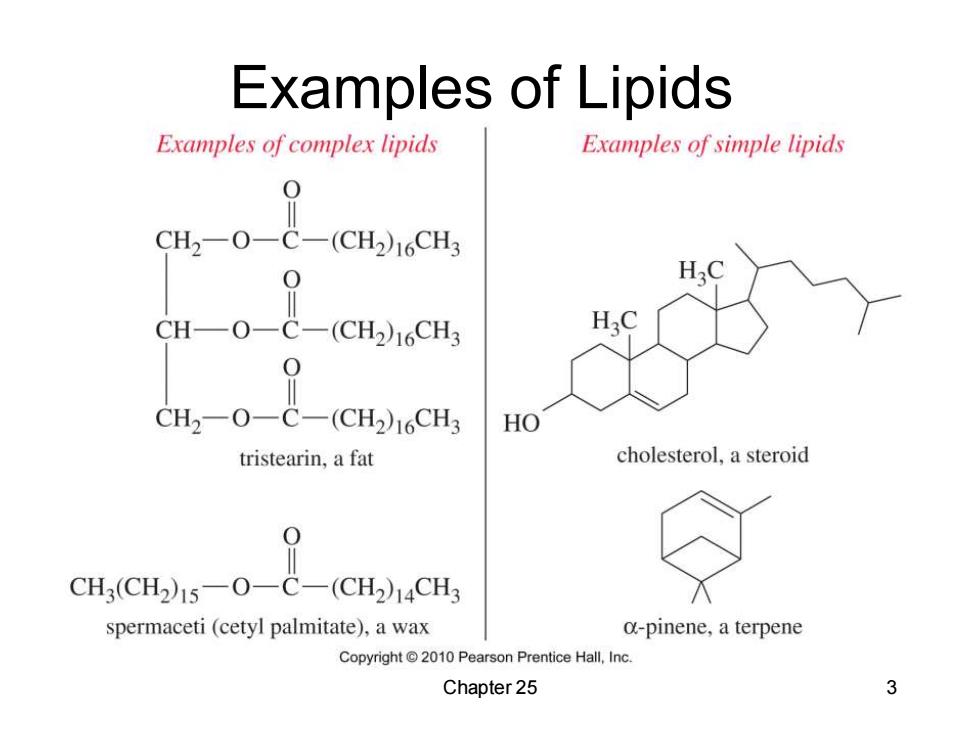
Examples of Lipids Examples of complex lipids Examples of simple lipids CH2-O-C-(CH2)16CH3 0 CH—O-C- (CH2)16CH3 CH2-O-C-(CH2)16CH3 HO tristearin,a fat cholesterol,a steroid CH3(CH2)15-O-C-(CH2)14CH3 spermaceti(cetyl palmitate),a wax a-pinene,a terpene Copyright2010 Pearson Prentice Hall,Inc. Chapter 25 3
Chapter 25 3 Examples of Lipids

Waxes Esters of long-chain fatty acids with long-chain alcohols. Commonly found in nature. Spermaceti is found in the head of the sperm whale. Beeswax is a mixture of waxes,hydrocarbons,and alcohols that bees used to make their honeycomb. Carnauba wax is a mixture of waxes of high molecular weights 0 CHa(CH)O- (CH2)24CH3 CH3(CH2)23O- (CH2)26CH3 a component of beeswax a component of carnauba wax Chapter 25
Chapter 25 4 Waxes ▪ Esters of long-chain fatty acids with long-chain alcohols. ▪ Commonly found in nature. ▪ Spermaceti is found in the head of the sperm whale. ▪ Beeswax is a mixture of waxes, hydrocarbons, and alcohols that bees used to make their honeycomb. ▪ Carnauba wax is a mixture of waxes of high molecular weights. CH3(CH2)29O C O (CH2)24CH3 CH3(CH2)23O C O (CH2)26CH3 a component of beeswax a component of carnauba wax

Fatty Acids Unbranched carboxylic acids with 12-20 carbons. Most contain an even number of carbons because they are built from acetic acid units. Melting points increase with increasing molecular weights. Unsaturation greatly lowers the melting point. Chapter 25 5
Chapter 25 5 Fatty Acids ▪ Unbranched carboxylic acids with 12–20 carbons. ▪ Most contain an even number of carbons because they are built from acetic acid units. ▪ Melting points increase with increasing molecular weights. ▪ Unsaturation greatly lowers the melting point