
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 16 Aromatic Compounds Copyright 2010 Pearson Education,Inc
Chapter 16 Aromatic Compounds Organic Chemistry, 7th Edition L. G. Wade, Jr. Copyright © 2010 Pearson Education, Inc

Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be CH6.He named it benzin. Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic. Chapter 16 2
Chapter 16 2 Discovery of Benzene • Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. • Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6 . He named it benzin. • Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic
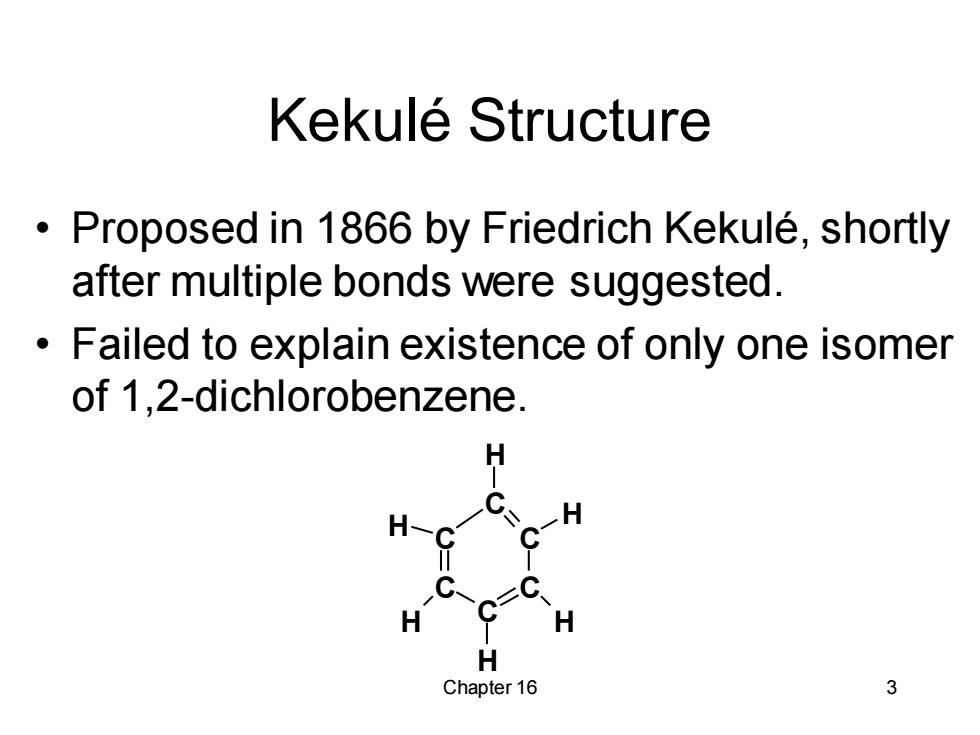
Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. H H-C H H H H Chapter 16 3
Chapter 16 3 Kekulé Structure • Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. • Failed to explain existence of only one isomer of 1,2-dichlorobenzene. C C C C C C H H H H H H
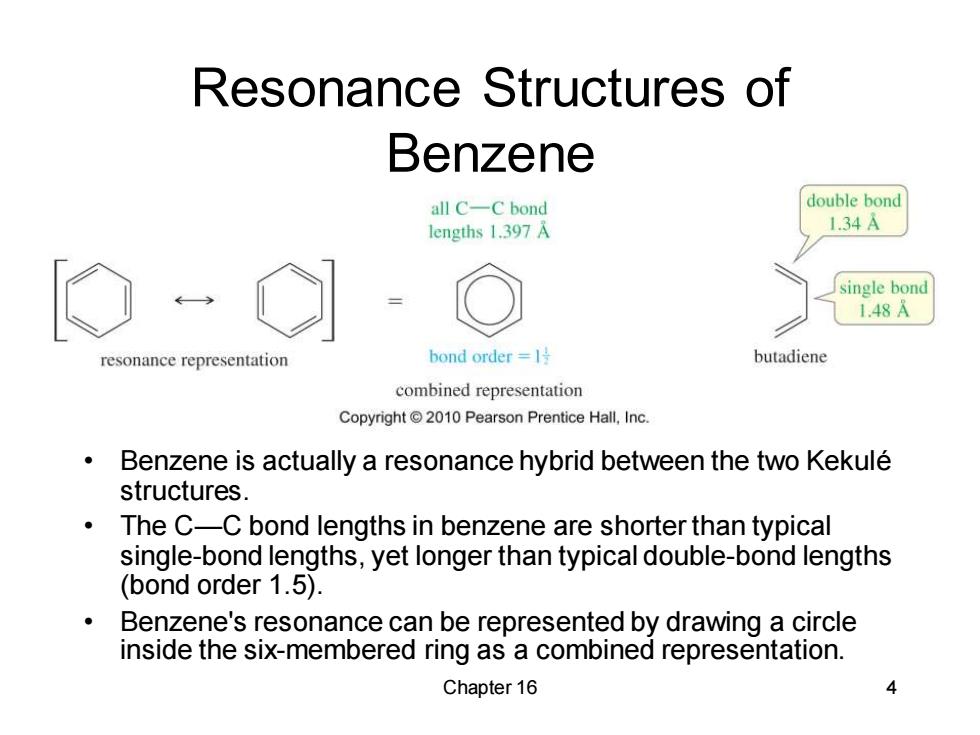
Resonance Structures of Benzene all C-C bond double bond lengths 1.397 A 1.34A single bond 1.48A resonance representation bond order=l号 butadiene combined representation Copyright 2010 Pearson Prentice Hall,Inc. Benzene is actually a resonance hybrid between the two Kekule structures. The C-C bond lengths in benzene are shorter than typical single-bond lengths,yet longer than typical double-bond lengths (bond order 1.5). Benzene's resonance can be represented by drawing a circle inside the six-membered ring as a combined representation. Chapter 16
Chapter 16 4 Resonance Structures of Benzene • Benzene is actually a resonance hybrid between the two Kekulé structures. • The C—C bond lengths in benzene are shorter than typical single-bond lengths, yet longer than typical double-bond lengths (bond order 1.5). • Benzene's resonance can be represented by drawing a circle inside the six-membered ring as a combined representation
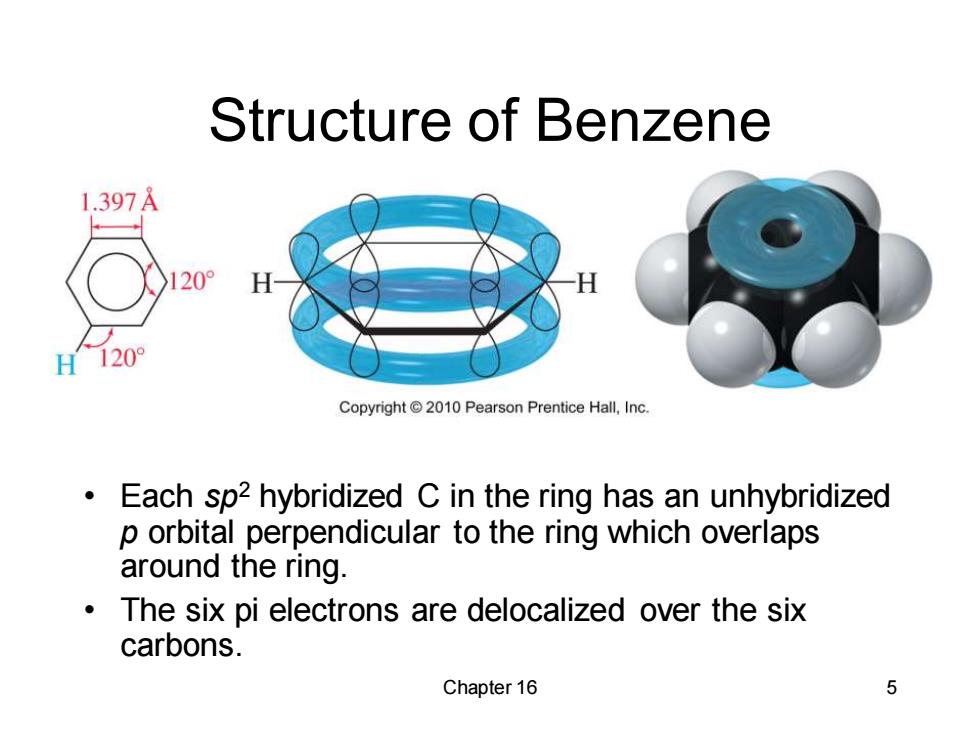
Structure of Benzene 1.397A 20° H 120° Copyright 2010 Pearson Prentice Hall,Inc. Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. The six pi electrons are delocalized over the six carbons. Chapter 16 5
Chapter 16 5 Structure of Benzene • Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. • The six pi electrons are delocalized over the six carbons