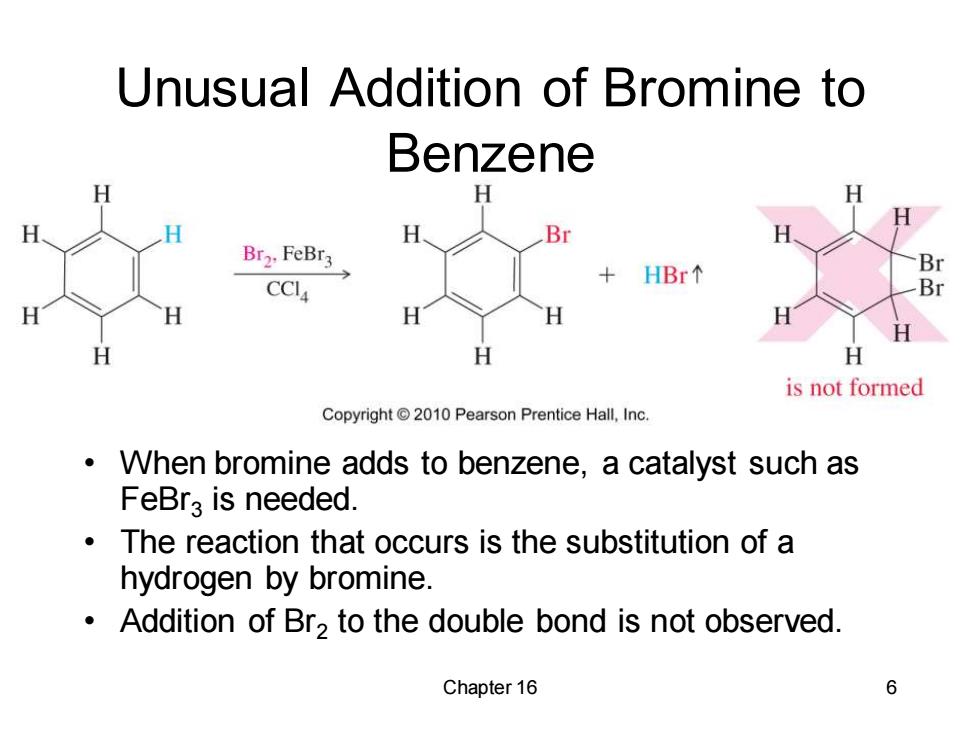
Unusual Addition of Bromine to Benzene H H H Br Br2.FeBr3 HBr Br CCI Br H H H H H is not formed Copyright2010 Pearson Prentice Hall,Inc. When bromine adds to benzene,a catalyst such as FeBra is needed. The reaction that occurs is the substitution of a hydrogen by bromine. Addition of Br2 to the double bond is not observed. Chapter 16 6
Chapter 16 6 Unusual Addition of Bromine to Benzene • When bromine adds to benzene, a catalyst such as FeBr3 is needed. • The reaction that occurs is the substitution of a hydrogen by bromine. • Addition of Br2 to the double bond is not observed

Resonance Energy Benzene does not have the predicted heat of hydrogenation of-359 kJ/mol. The observed heat of hydrogenation is -208 kJ/mol,a difference of 151 kJ. This difference between the predicted and the observed value is called the resonance energy. Chapter 16 7
Chapter 16 7 Resonance Energy • Benzene does not have the predicted heat of hydrogenation of -359 kJ/mol. • The observed heat of hydrogenation is -208 kJ/mol, a difference of 151 kJ. • This difference between the predicted and the observed value is called the resonance energy
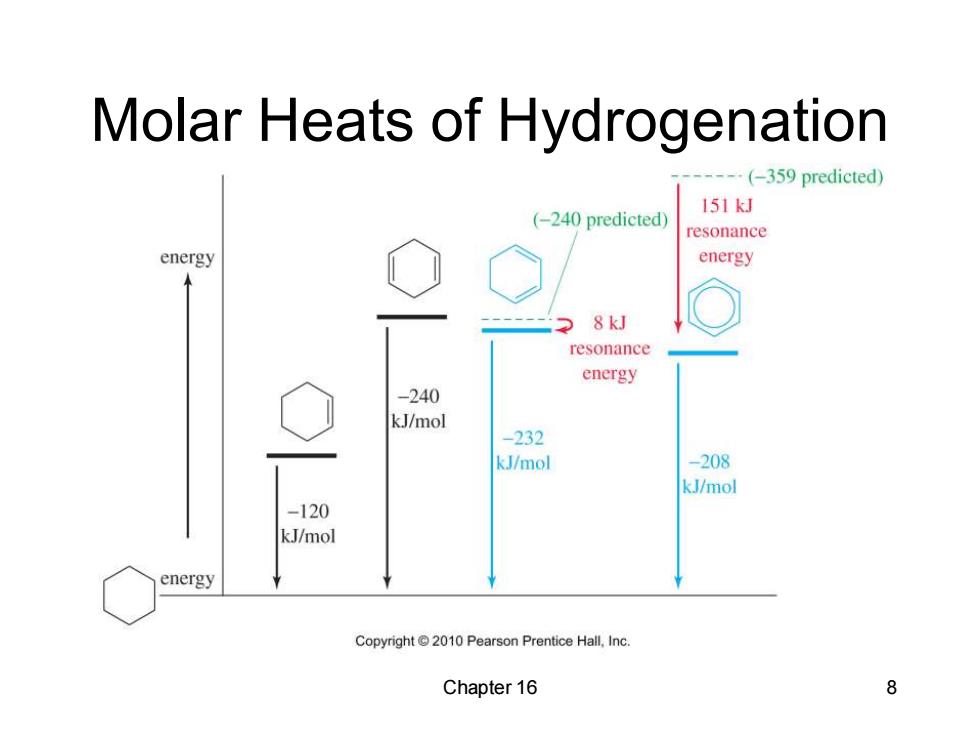
Molar Heats of Hydrogenation ---(-359 predicted) (-240 predicted) 151kJ resonance energy energy 2 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy Copyright 2010 Pearson Prentice Hall,Inc. Chapter 16 8
Chapter 16 8 Molar Heats of Hydrogenation

Annulenes cyclobutadiene benzene cyclooctatetraene cyclodecapentaene [4]annulene [6]annulene [8]annulene [10]annulene Copyright 2010 Pearson Prentice Hall,Inc. Annulenes are hydrocarbons with alternating single and double bonds. Benzene is a six-membered annulene,so it can be named [6]-annulene.Cylobutadiene is [4]-annulene, cyclooctatetraene is [8]-annulene. Chapter 16 9
Chapter 16 9 Annulenes • Annulenes are hydrocarbons with alternating single and double bonds. • Benzene is a six-membered annulene, so it can be named [6]-annulene. Cylobutadiene is [4]-annulene, cyclooctatetraene is [8]-annulene
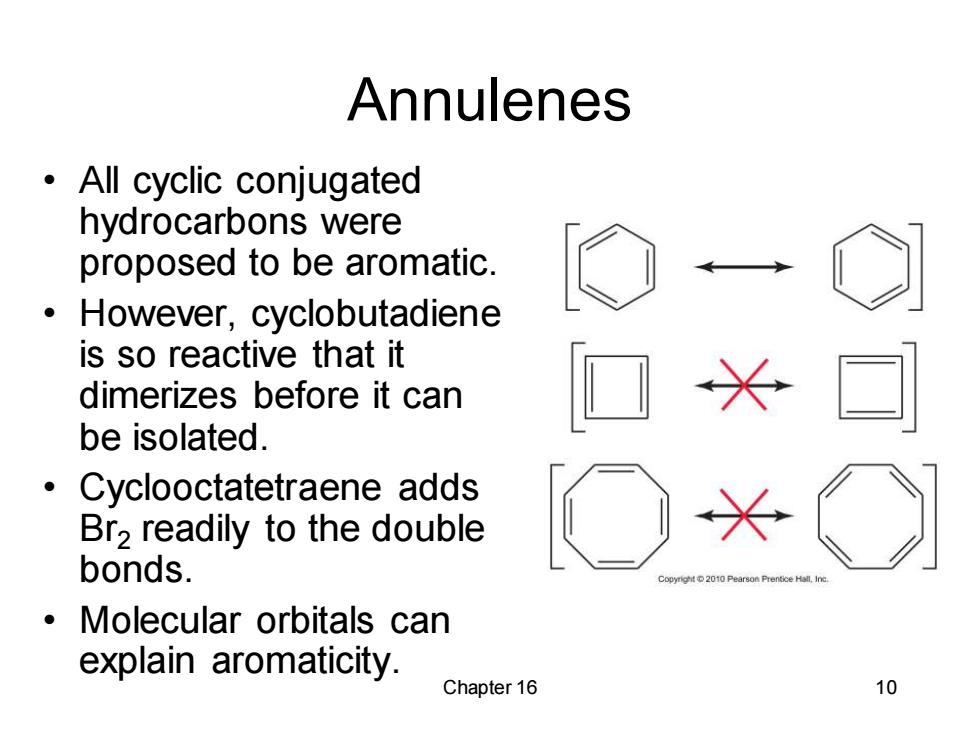
Annulenes All cyclic conjugated hydrocarbons were proposed to be aromatic. However,cyclobutadiene is so reactive that it dimerizes before it can be isolated. Cyclooctatetraene adds Br2 readily to the double bonds. Molecular orbitals can explain aromaticity. Chapter 16 10
Chapter 16 10 Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • Cyclooctatetraene adds Br2 readily to the double bonds. • Molecular orbitals can explain aromaticity