Organic Chemistry,7th Edition 1.4A 0.96A L.G.Wade,Jr. 108.9 HH Chapter 10 Structure and Synthesis of Alcohols Copyright 2010 Pearson Education,Inc
Chapter 10 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Structure and Synthesis of Alcohols
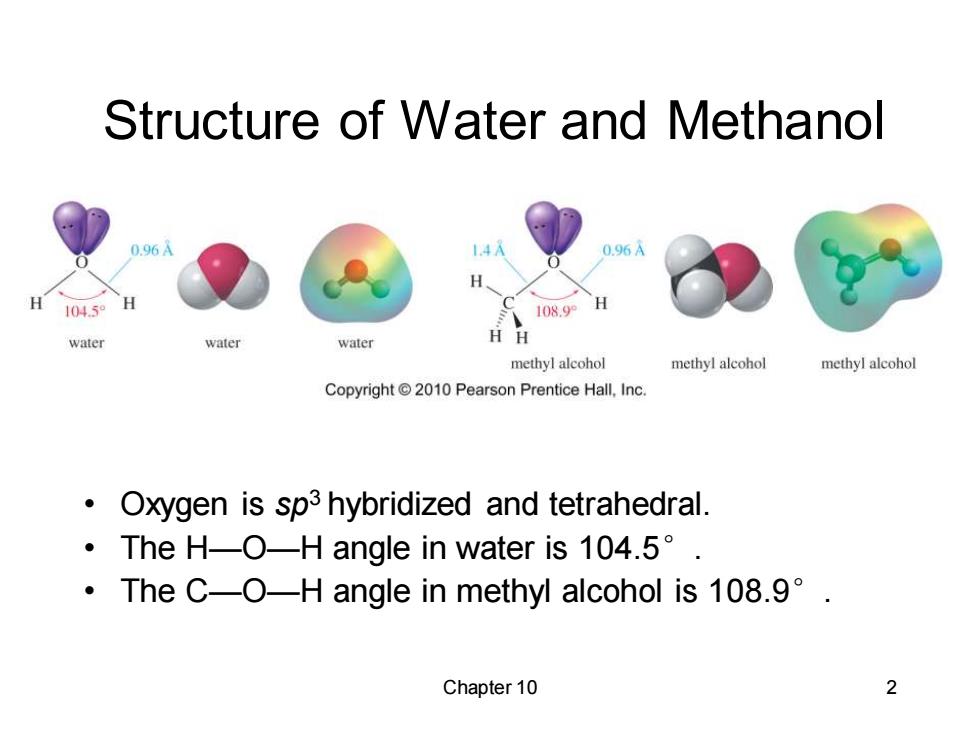
Structure of Water and Methanol 0.96A 1.4A 0.96A H 1045 H 108.9 H water water water H methyl alcohol methyl alcohol methyl alcohol Copyright2010 Pearson Prentice Hall,Inc. Oxygen is sp3 hybridized and tetrahedral. ·TheH-O-H angle in water is104.5°. ·TheC-O-H angle in methyl alcohol is108.9°. Chapter 10
Chapter 10 2 Structure of Water and Methanol • Oxygen is sp3 hybridized and tetrahedral. • The H—O—H angle in water is 104.5°. • The C—O—H angle in methyl alcohol is 108.9°

Classification of Alcohols Primary:carbon with-OH is bonded to one other carbon. Secondary:carbon with-OH is bonded to two other carbons. Tertiary:carbon with -OH is bonded to three other carbons. Aromatic (phenol):-OH is bonded to a benzene ring. Chapter 10 3
Chapter 10 3 Classification of Alcohols • Primary: carbon with —OH is bonded to one other carbon. • Secondary: carbon with —OH is bonded to two other carbons. • Tertiary: carbon with —OH is bonded to three other carbons. • Aromatic (phenol): —OH is bonded to a benzene ring

Examples of Classifications CH3 QH CH3-CH-CH2OH CH3-CH-CH2CH3 Primary alcohol Secondary alcohol CH3 CH3 C-oh Tertiary alcohol CH3 Chapter 10 4
Chapter 10 4 Examples of Classifications CH3 C CH3 CH3 * OH CH3 CH OH CH2CH3 * CH3 CH CH3 CH2OH * Primary alcohol Secondary alcohol Tertiary alcohol

IUPAC Nomenclature Find the longest carbon chain containing the carbon with the-OH group. Drop the -e from the alkane name,add -ol. Number the chain giving the-OH group the lowest number possible. Number and name all substituents and write them in alphabetical order. Chapter 10 5
Chapter 10 5 IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the —OH group. • Drop the -e from the alkane name, add -ol. • Number the chain giving the —OH group the lowest number possible. • Number and name all substituents and write them in alphabetical order