
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 3 Structure and Stereochemistry of Alkanes Copyright 2010 Pearson Education,Inc. Chapter 3 1
Chapter 3 1 Chapter 3 Organic Chemistry, 7th Edition L. G. Wade, Jr. Copyright © 2010 Pearson Education, Inc. Structure and Stereochemistry of Alkanes

Hydrocarbons Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. TABLE 3-1 Hydrocarbon Classifications Compound Type Functional Group Example alkanes none (no double or triple bonds) CH3-CH2-CH3,propane alkenes C=C double bond CH2=CH-CH3.propene alkynes -C=C-triple bond H-C=C-CH3.propyne CHCH, aromatics benzene ring ethylbenzene Copyright 2010 Pearson Prentice Hall,Inc. Chapter 3 2
Chapter 3 2 Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. Hydrocarbons
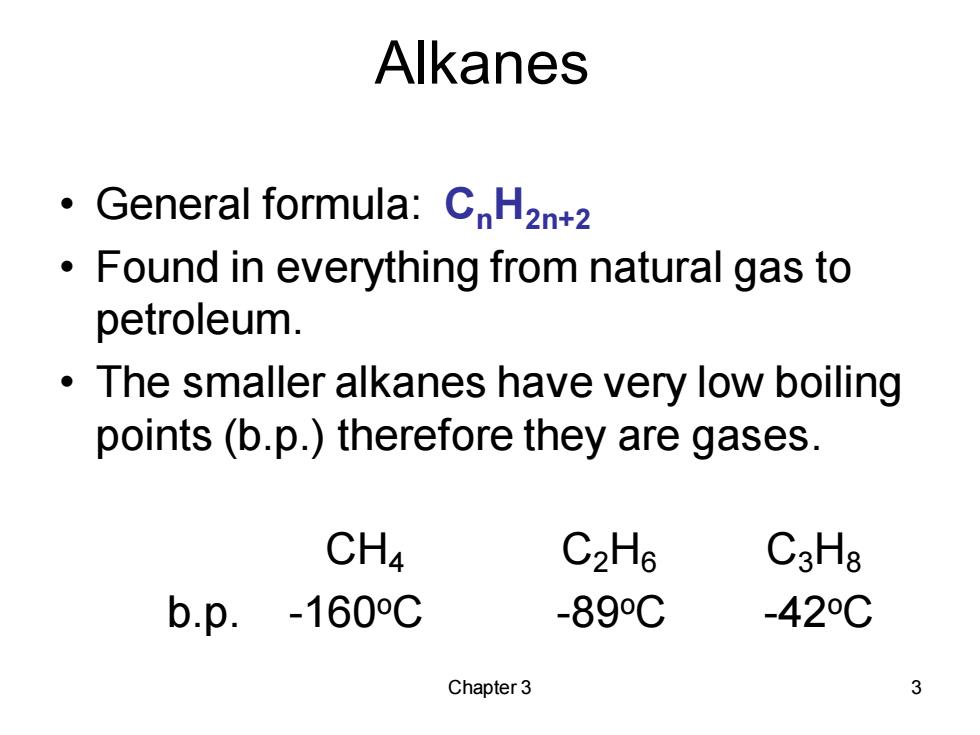
Alkanes General formula:CH2n+2 Found in everything from natural gas to petroleum. The smaller alkanes have very low boiling points (b.p.)therefore they are gases. CH4 C2H6 C3Hg b.p.-160C -89C -42C Chapter 3 3
Chapter 3 3 Alkanes • General formula: CnH2n+2 • Found in everything from natural gas to petroleum. • The smaller alkanes have very low boiling points (b.p.) therefore they are gases. CH4 C2H6 C3H8 b.p. -160oC -89oC -42oC
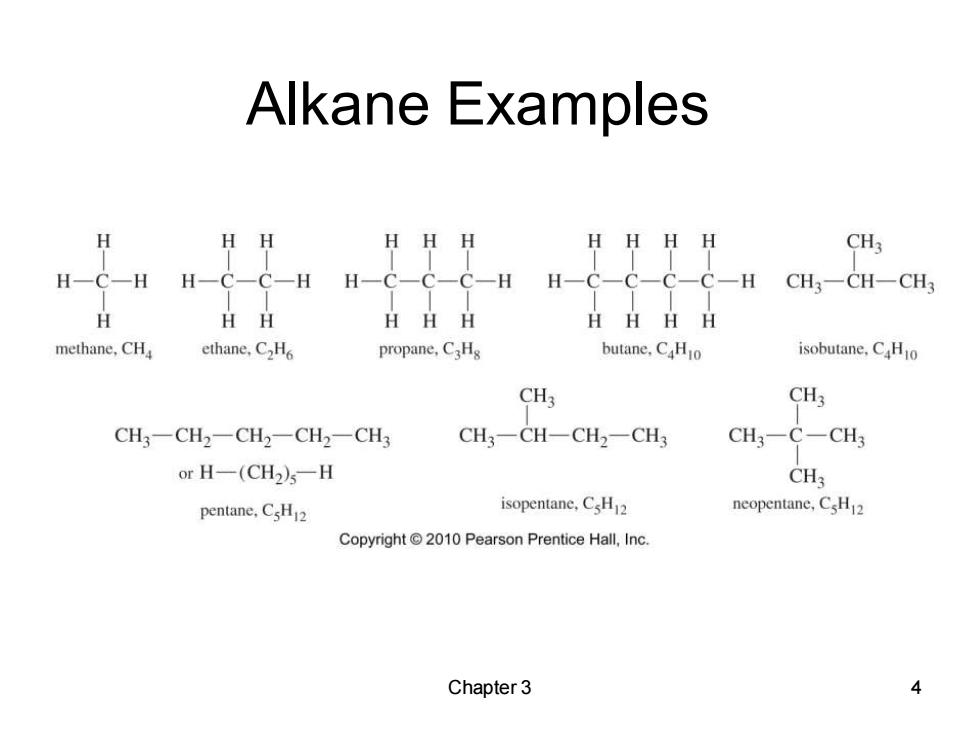
Alkane Examples H H HHH HHHH CH3 H-C-C-C-C-H CH3-CH-CH3 HH HHH HHHH methane,CHa ethane,C2H propane.C3Hs butane.CHio isobutane,CHo CH3 CH3 CH3-CH2一CH2-CH2-CH3 CH3一CH-CH2-CH CH3-C-CH3 or H-(CH2)s-H CH3 pentane.CsH2 isopentane,CsH2 neopentane,CsH2 Copyright 2010 Pearson Prentice Hall,Inc. Chapter 3 4
Chapter 3 4 Alkane Examples

Small Alkanes(CnH2n+2) ·Methane CH4 Ethane CH3-CH3 。Propane CH3-CH2-CH3 Chapter 3 5
Chapter 3 5 Small Alkanes (CnH2n+2) • Methane • Ethane • Propane CH3 CH3 CH3 CH2 CH3 CH4