
E Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
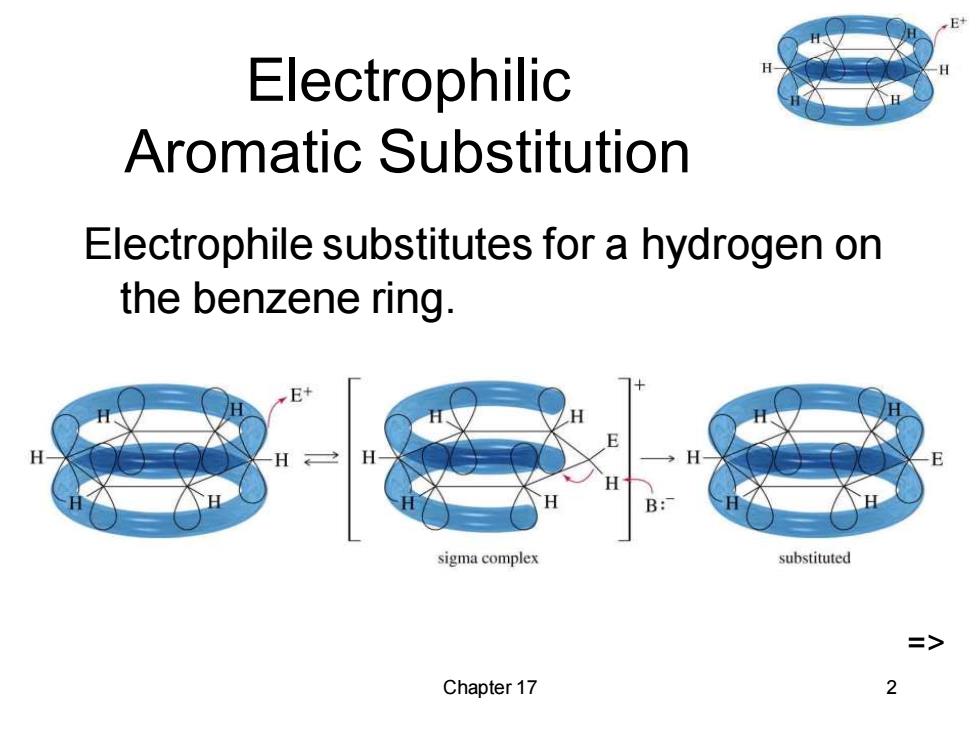
Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. sigma complex substituted => Chapter 17 2
Chapter 17 2 Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. =>
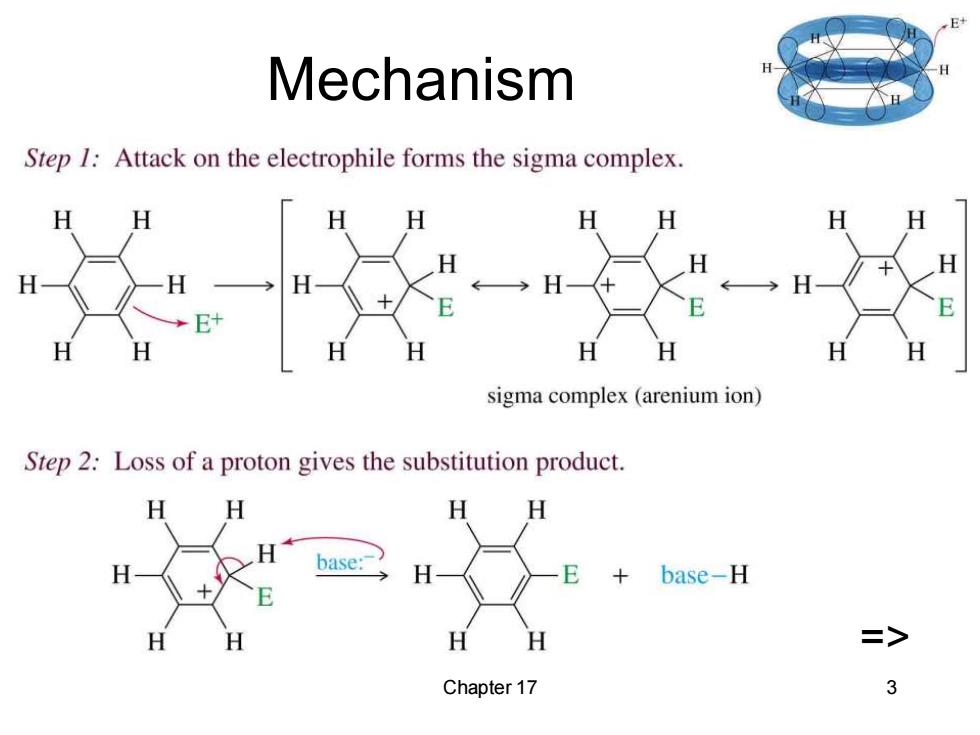
E+ Mechanism Step I:Attack on the electrophile forms the sigma complex. E sigma complex(arenium ion) Step 2:Loss of a proton gives the substitution product. H H base E base-H H H Chapter 17 3
Chapter 17 3 Mechanism =>
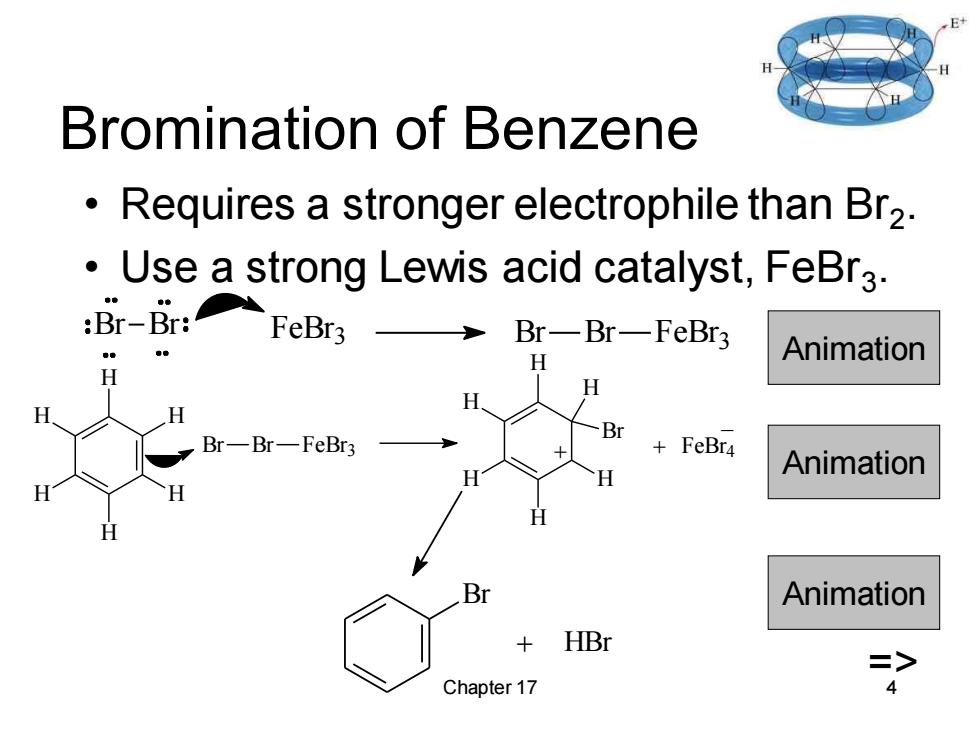
H Bromination of Benzene Requires a stronger electrophile than Br2. Use a strong Lewis acid catalyst,FeBr3. :Br-Br:FeBr3→ Br-Br—FeBr3 H Animation H H H H H Br Br-Br-FeBr3 +FeBr4 H Animation H Animation + HBr => Chapter 17
Chapter 17 4 Bromination of Benzene • Requires a stronger electrophile than Br2 . • Use a strong Lewis acid catalyst, FeBr3 . Br Br FeBr 3 Br Br FeBr 3 Animation Br Br FeBr3 H H H H H H H H H H H H Br + + FeBr4 _ Animation Br Animation + HBr =>
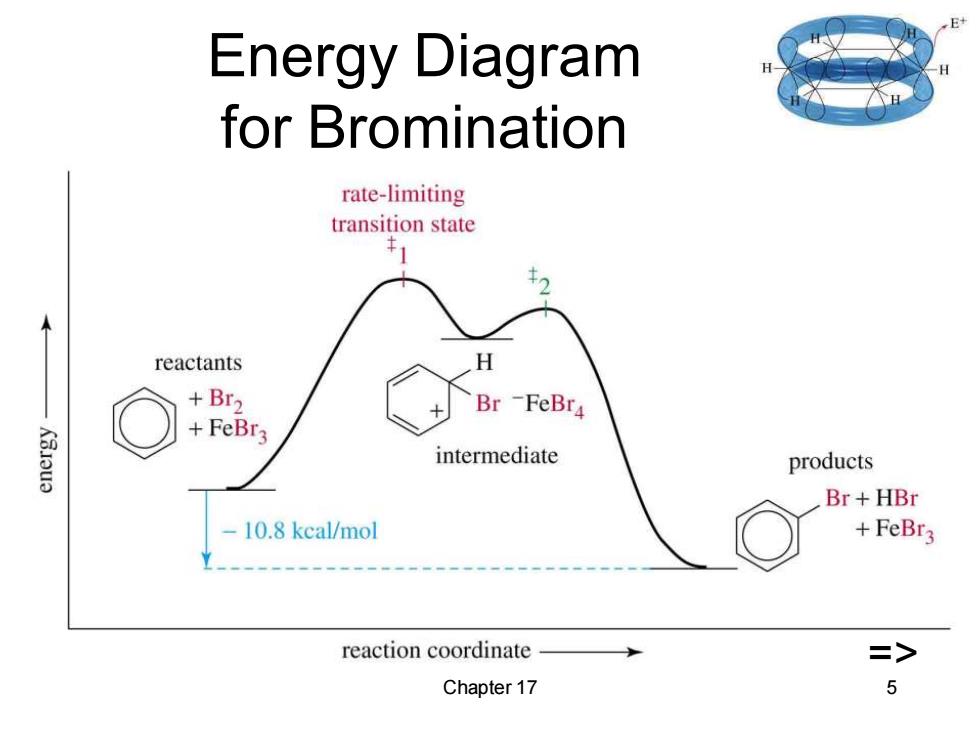
Energy Diagram for Bromination rate-limiting transition state reactants H +Br2 Br -FeBr4 +FeBr3 intermediate products Br +HBr 10.8 kcal/mol +FeBr3 reaction coordinate => Chapter 17 5
Chapter 17 5 Energy Diagram for Bromination =>