
CH:CH2CH2 Organic Chemistry,5th Edition H HH L.G.Wade,Jr. Chapter 11 Reactions of Alcohols Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
CH3CH2CH2 C H H Br O H H Chapter 11 Reactions of Alcohols Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

CH3CH2CH2 H Types of Alcohol Reactions HH Dehydration to alkene Oxidation to aldehyde,ketone Substitution to form alkyl halide ·Reduction to alkane ·Esterification ·Tosylation Williamson synthesis of ether => Chapter 11 2
CH3CH2CH2 C H H Br O H H Chapter 11 2 Types of Alcohol Reactions • Dehydration to alkene • Oxidation to aldehyde, ketone • Substitution to form alkyl halide • Reduction to alkane • Esterification • Tosylation • Williamson synthesis of ether =>
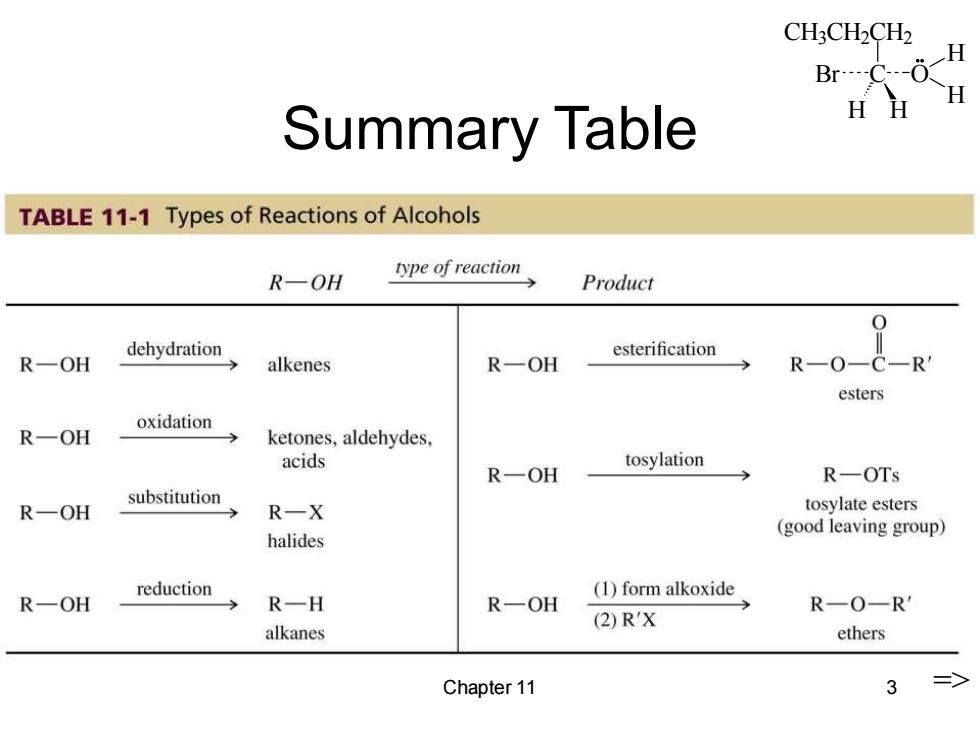
CH3CH2CH2 H Br…-C-( Summary Table HH H TABLE 11-1 Types of Reactions of Alcohols type of reaction R-OH Product dehydration esterification R一OH alkenes R一OH R-0-C-R esters oxidation R一OH ketones,aldehydes, acids tosylation R一OH R-OTs substitution R一OH R一X tosylate esters halides (good leaving group) reduction (1)form alkoxide R—OH R一H R一OH R-0-R' (2)RX alkanes ethers Chapter 11 3
CH3CH2CH2 C H H Br O H H Chapter 11 3 Summary Table =>
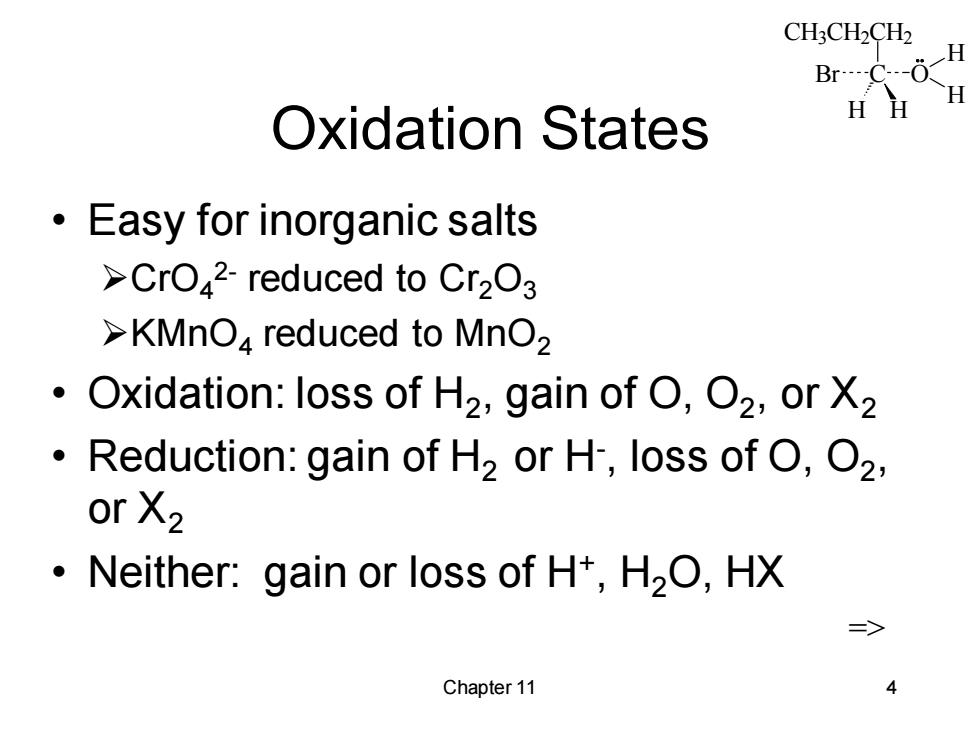
CH3CH2CH2 H Br----C--- H Oxidation States HH Easy for inorganic salts >CrO42-reduced to Cr2O3 >KMnO reduced to MnO2 Oxidation:loss of H2,gain of O,O2,or X2 Reduction:gain of H2 or H,loss of O,O2, or X2 Neither:gain or loss of H+,H2O,HX => Chapter 11 4
CH3CH2CH2 C H H Br O H H Chapter 11 4 Oxidation States • Easy for inorganic salts ➢CrO4 2- reduced to Cr2O3 ➢KMnO4 reduced to MnO2 • Oxidation: loss of H2 , gain of O, O2 , or X2 • Reduction: gain of H2 or H- , loss of O, O2 , or X2 • Neither: gain or loss of H+ , H2O, HX =>
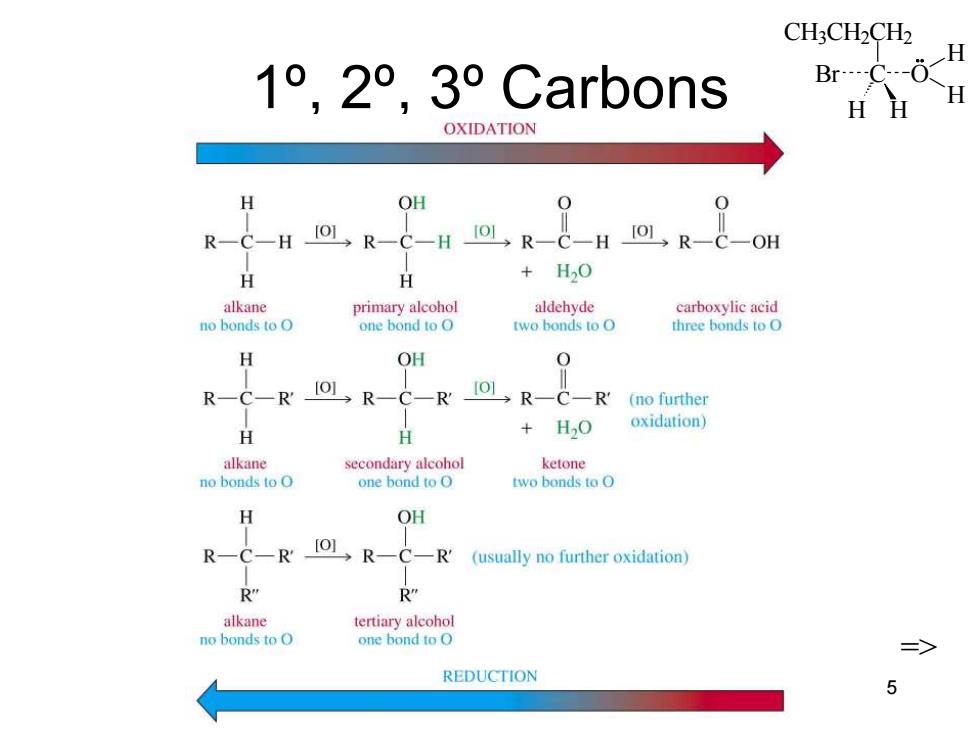
CH;CH2CH2 H 1°,2°,3°Carb0ns Br----C---( -H HH OXIDATION H OH R-C-HOR-&-H回R-C-H回R-C-OH H H +H2O alkane primary alcohol aldehyde carboxylic acid no bonds to O one bond to O two bonds to O three bonds to O H OH RCRIOL R-CR可RC (no further +H2O oxidation) H alkane secondary alcohol ketone no bonds to O one bond to O two bonds to O H OH R—CR R-C—R' (usually no further oxidation) R" R" alkane tertiary alcohol no bonds to O one bond to O REDUCTION 5
CH3CH2CH2 C H H Br O H H Chapter 11 5 1º, 2º, 3º Carbons =>