
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 15 Conjugated Systems, Orbital Symmetry,and Ultraviolet Spectroscopy Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
Definitions Conjugated double bonds are separated by one single bond.Example:1,3-pentadiene. Isolated double bonds are separated by two or more single bonds.1,4-pentadiene. Cumulated double bonds are on adjacent carbons.Example:1,2-pentadiene. 二> Chaper 15 2
Chaper 15 2 Definitions • Conjugated double bonds are separated by one single bond. Example: 1,3-pentadiene. • Isolated double bonds are separated by two or more single bonds. 1,4-pentadiene. • Cumulated double bonds are on adjacent carbons. Example: 1,2-pentadiene. =>
Resonance Energy Heat of hydrogenation for trans-1,3- pentadiene is less than expected. ·△Hfor1-pentene is30.0kcal/mol and for trans-2-pentene is 27.4 kcal/mol,so expect 57.4 kcal for trans-1,3-pentadiene. ·Actual△His53.7kcal,so the conjugated diene is more stable. Difference,(57.4-53.7)3.7 kcal/mol,is the resonance energy. => Chaper 15 3
Chaper 15 3 Resonance Energy • Heat of hydrogenation for trans-1,3- pentadiene is less than expected. • H for 1-pentene is 30.0 kcal/mol and for trans-2-pentene is 27.4 kcal/mol, so expect 57.4 kcal for trans-1,3-pentadiene. • Actual H is 53.7 kcal, so the conjugated diene is more stable. • Difference, (57.4 – 53.7) 3.7 kcal/mol, is the resonance energy. =>
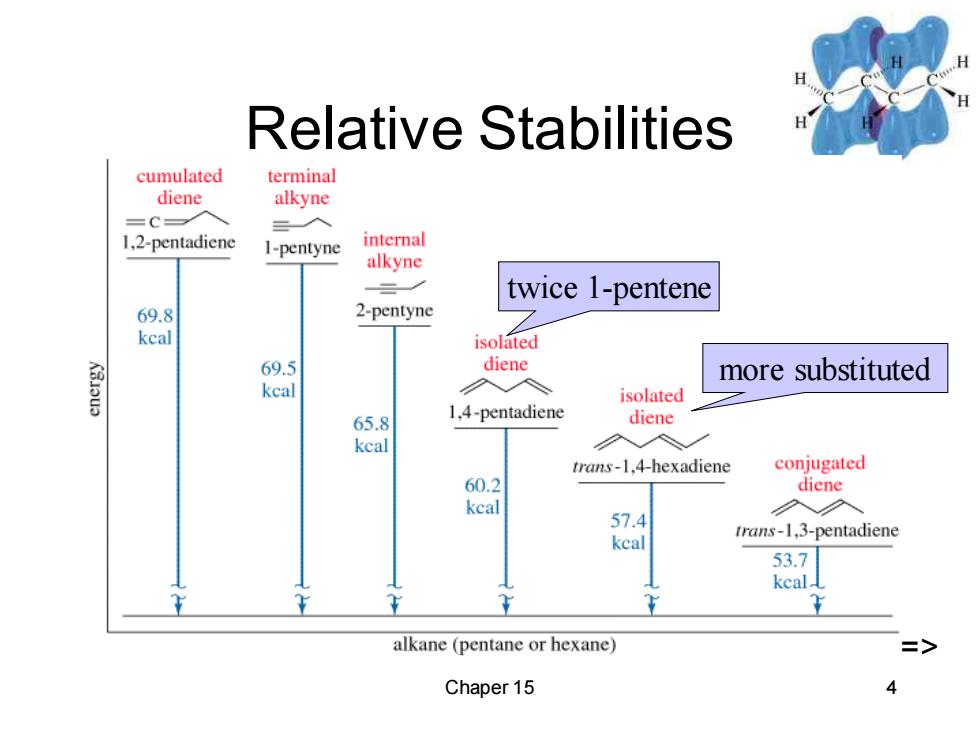
Relative Stabilities cumulated terminal diene alkyne =C- 入 1,2-pentadiene 1I-pentyne internal alkyne twice 1-pentene 69.8 2-pentyne kcal isolated 69.5 diene more substituted kcal isolated 65.8 1,4-pentadiene diene kcal trans-1,4-hexadiene conjugated 60.2 diene keal 57.4 trans-1,3-pentadiene kcal 53.7 kcal alkane (pentane or hexane) Chaper 15 4
Chaper 15 4 Relative Stabilities twice 1-pentene more substituted =>
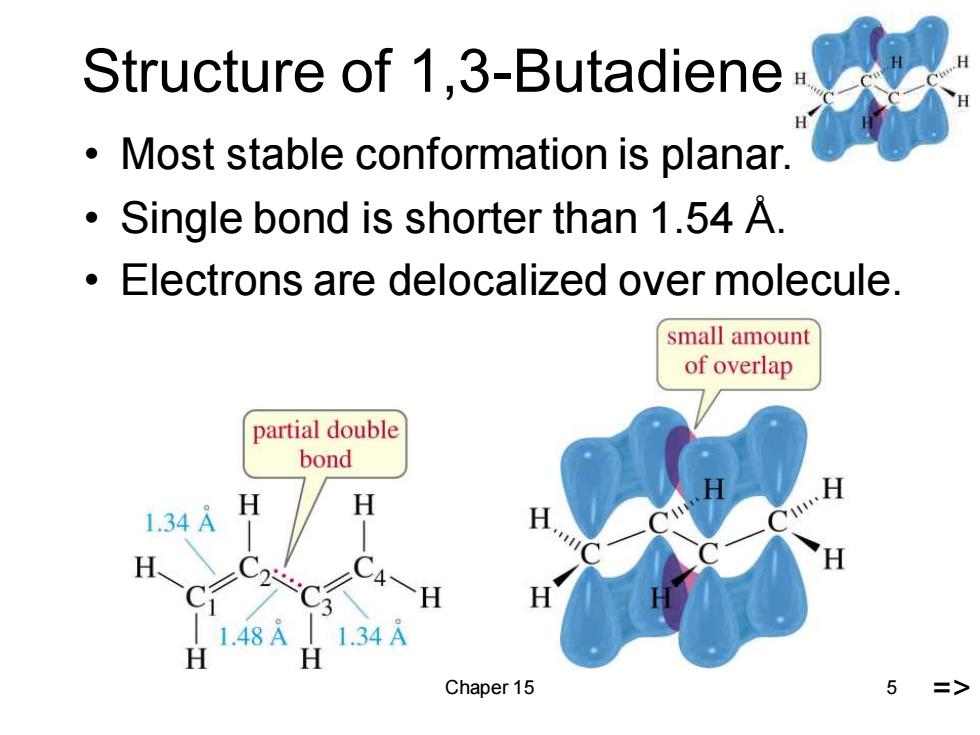
Structure of 1,3-Butadiene Most stable conformation is planar. Single bond is shorter than 1.54 A. Electrons are delocalized over molecule. small amount of overlap partial double bond 1.34A H H 1.48A 11.34A H Chaper 15 5
Chaper 15 5 Structure of 1,3-Butadiene • Most stable conformation is planar. • Single bond is shorter than 1.54 Å. • Electrons are delocalized over molecule. =>