
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 6 Alkyl Halides:Nucleophilic Substitution and Elimination Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©20o3,Prentice Hall
Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination Organic Chemistry, 5th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall
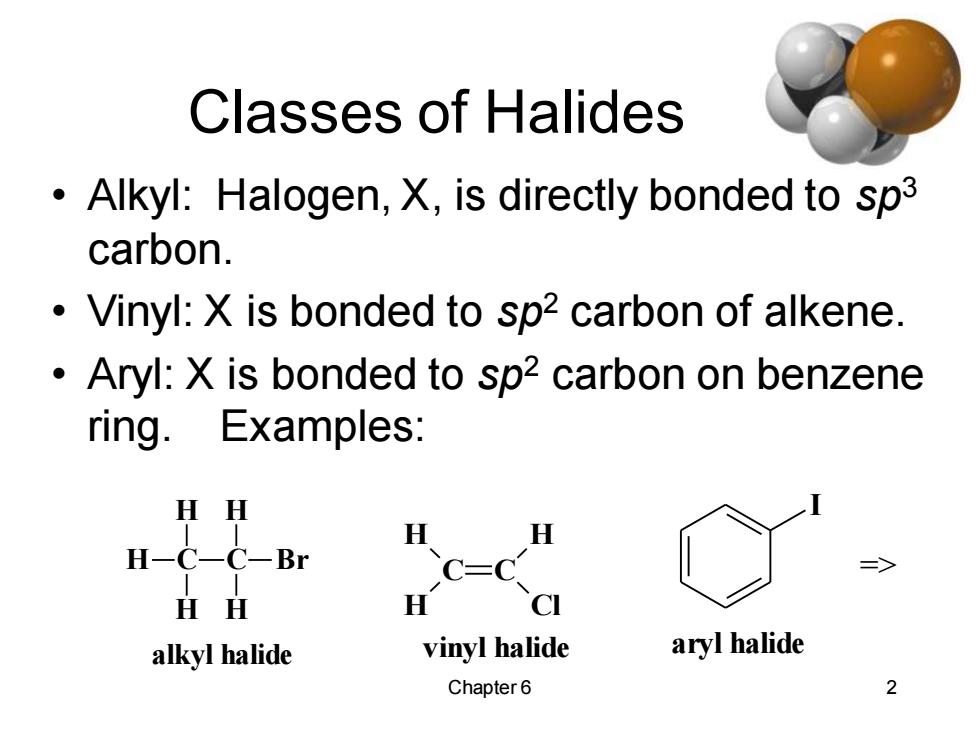
Classes of Halides Alkyl:Halogen,X,is directly bonded to sp3 carbon. Vinyl:X is bonded to sp2 carbon of alkene. Aryl:X is bonded to sp2 carbon on benzene ring.Examples: HH H H H-C一C-Br C=C HH H CI alkyl halide vinyl halide aryl halide Chapter 6 2
Chapter 6 2 Classes of Halides • Alkyl: Halogen, X, is directly bonded to sp3 carbon. • Vinyl: X is bonded to sp2 carbon of alkene. • Aryl: X is bonded to sp2 carbon on benzene ring. Examples: C H H H C H H Br alkyl halide C C H H H Cl vinyl halide I aryl halide =>
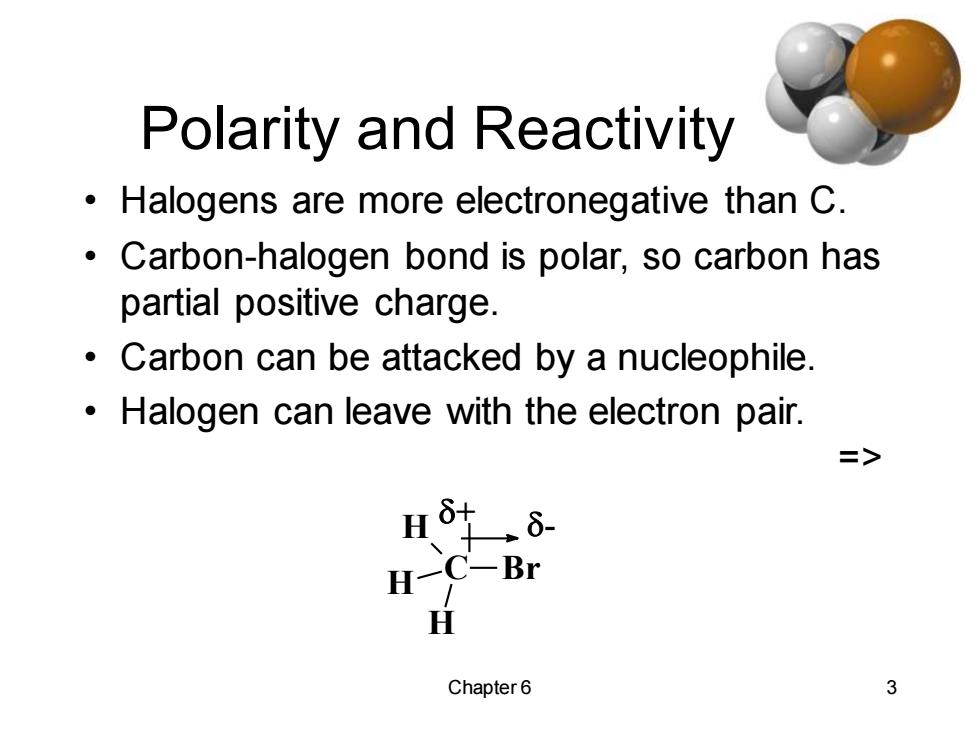
Polarity and Reactivity Halogens are more electronegative than C. Carbon-halogen bond is polar,so carbon has partial positive charge. Carbon can be attacked by a nucleophile Halogen can leave with the electron pair. => H H一 C-Br H Chapter6 3
Chapter 6 3 Polarity and Reactivity • Halogens are more electronegative than C. • Carbon-halogen bond is polar, so carbon has partial positive charge. • Carbon can be attacked by a nucleophile. • Halogen can leave with the electron pair. => C H H H Br + -
Classes of Alkyl Halides Methyl halides:only one C,CHaX Primary:C to which X is bonded has only one C-C bond. Secondary:C to which X is bonded has two C-C bonds. Tertiary:C to which X is bonded has three C-C bonds. > Chapter 6
Chapter 6 4 Classes of Alkyl Halides • Methyl halides: only one C, CH3X • Primary: C to which X is bonded has only one C-C bond. • Secondary: C to which X is bonded has two C-C bonds. • Tertiary: C to which X is bonded has three C-C bonds. =>
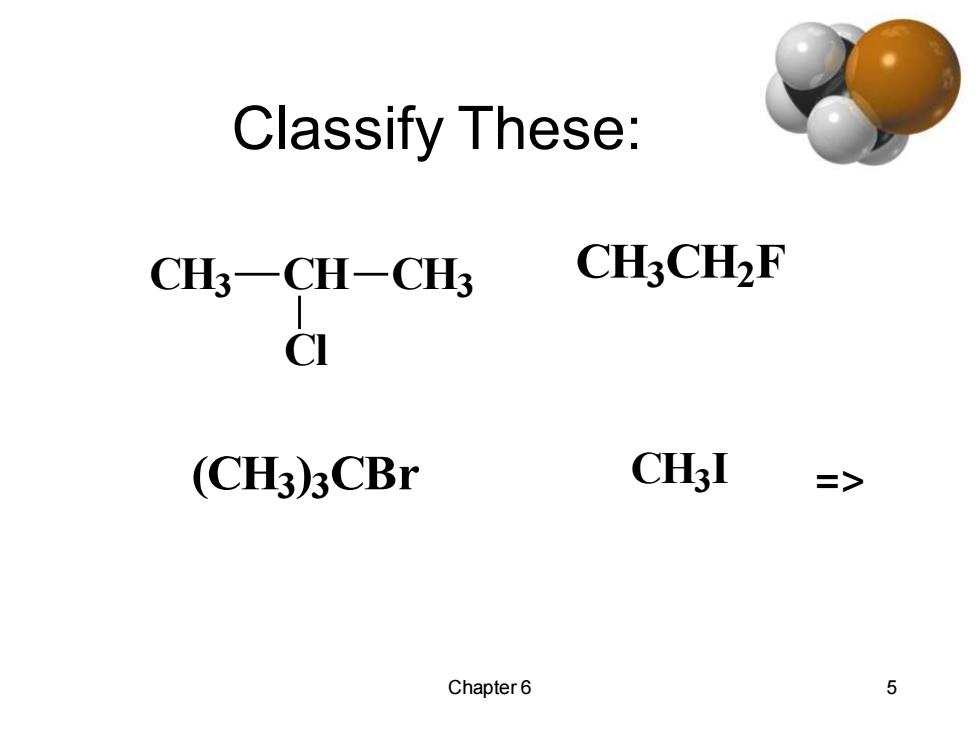
Classify These: CH3-CH-CH3 CH3CH2F CI (CH3)3CBr CH3I => Chapter6 5
Chapter 6 5 Classify These: CH3 CH CH3 Cl CH3 CH2 F (CH3) 3 CBr CH3 I =>