
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 12 Infrared Spectroscopy and Mass Spectrometry Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 12 Infrared Spectroscopy and Mass Spectrometry Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength is varied. Chapter 12
Chapter 12 2 Introduction • Spectroscopy is an analytical technique which helps determine structure. • It destroys little or no sample. • The amount of light absorbed by the sample is measured as wavelength is varied. =>
Types of Spectroscopy Infrared (IR)spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. Mass spectrometry (MS)fragments the molecule and measures the masses. Nuclear magnetic resonance (NMR) spectroscopy detects signals from hydrogen atoms and can be used to distinguish isomers. Ultraviolet (UV)spectroscopy uses electron transitions to determine bonding patterns.= Chapter 12 3
Chapter 12 3 Types of Spectroscopy • Infrared (IR) spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. • Mass spectrometry (MS) fragments the molecule and measures the masses. • Nuclear magnetic resonance (NMR) spectroscopy detects signals from hydrogen atoms and can be used to distinguish isomers. • Ultraviolet (UV) spectroscopy uses electron transitions to determine bonding patterns. =>
Electromagnetic Spectrum Examples:X rays,microwaves,radio waves,visible light,IR,and UV. Frequency and wavelength are inversely proportional. .c=Av,where c is the speed of light. Energy per photon hv,where h is Planck's constant. Chapter 12
Chapter 12 4 Electromagnetic Spectrum • Examples: X rays, microwaves, radio waves, visible light, IR, and UV. • Frequency and wavelength are inversely proportional. • c = ln, where c is the speed of light. • Energy per photon = hn, where h is Planck’s constant. =>
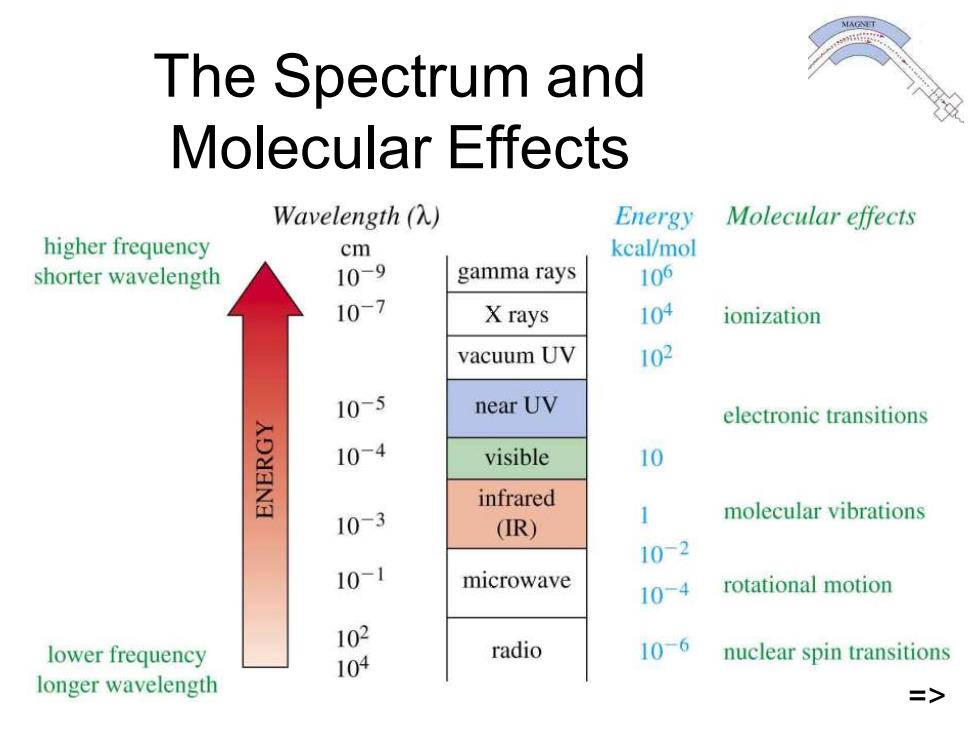
The Spectrum and Molecular Effects Wavelength(A) Energy Molecular effects higher frequency cm kcal/mol shorter wavelength 109 gamma rays 106 107 X rays 104 ionization vacuum UV 102 10-5 near UV electronic transitions 10-4 visible 10 infrared 10-3 molecular vibrations (R) 1 10-2 101 microwave 10-4 rotational motion 102 lower frequency radio 104 10-6 nuclear spin transitions longer wavelength =>
Chapter 12 5 The Spectrum and Molecular Effects => =>