
PhOCH, H Organic Chemistry,5th Edition L.G.Wade,Jr. COOH insctive Chapter 21 Carboxylic Acid Derivatives Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 21 Carboxylic Acid Derivatives Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
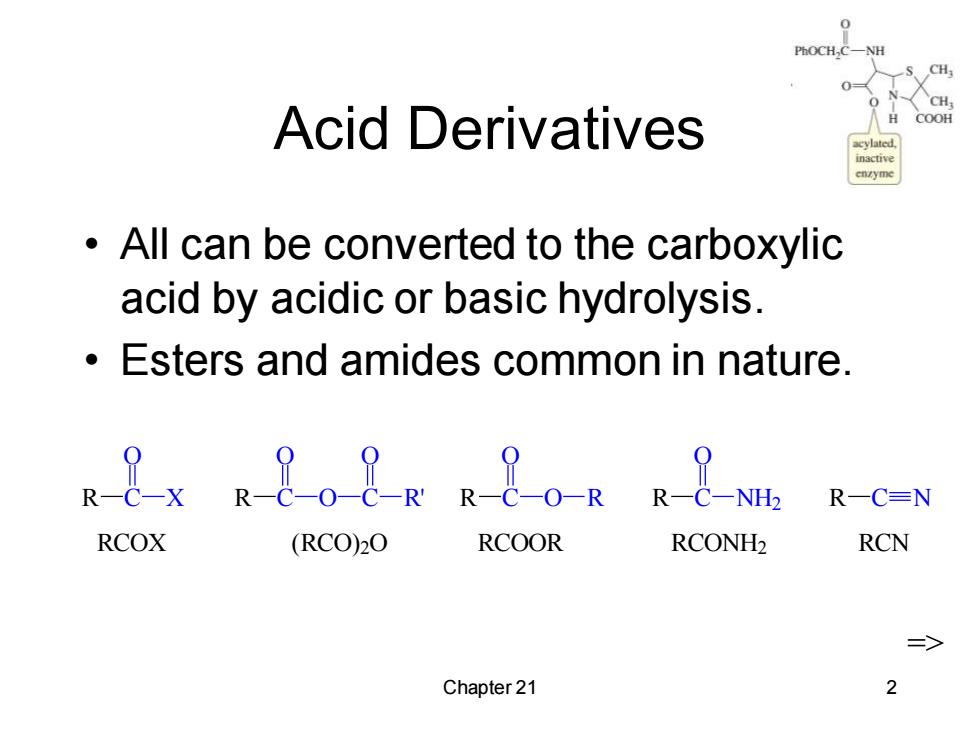
PhOCH, H Acid Derivatives COOH All can be converted to the carboxylic acid by acidic or basic hydrolysis. Esters and amides common in nature. R8xR8oQRR8。gR8 ,R一C=N RCOX (RCO)20 RCOOR RCONH2 RCN Chapter 21 2
Chapter 21 2 Acid Derivatives • All can be converted to the carboxylic acid by acidic or basic hydrolysis. • Esters and amides common in nature. => R C O X R C O O C O R' R C O O R R C O NH2 R C N RCOX (RCO)2O RCOOR RCONH2 RCN
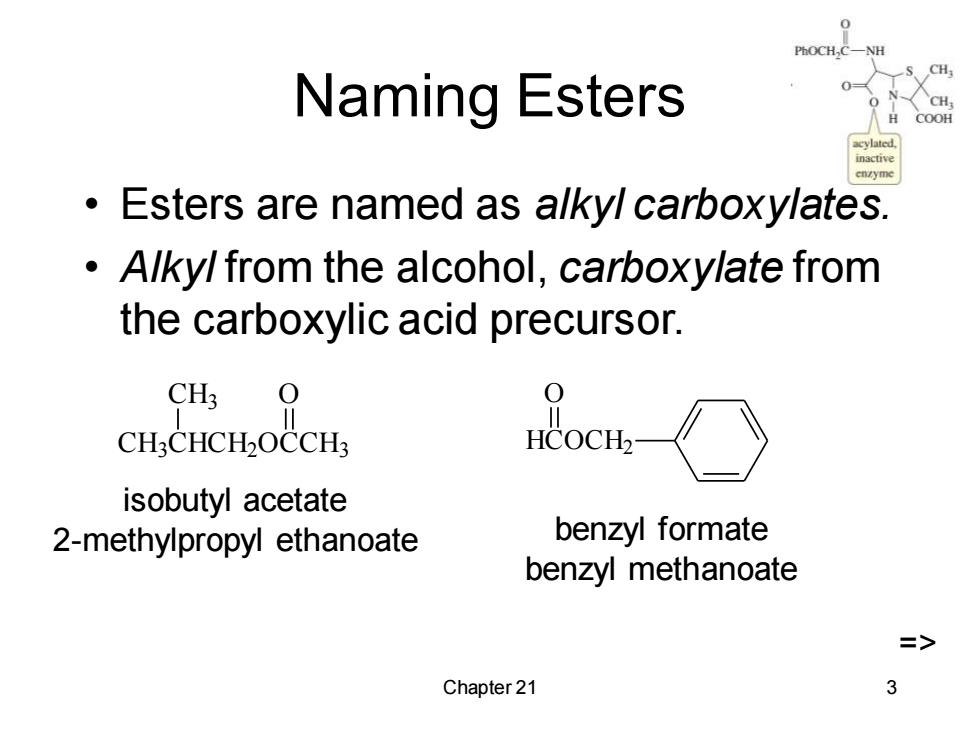
H Naming Esters COOH inactive Esters are named as alkyl carboxylates. Alky/from the alcohol,carboxylate from the carboxylic acid precursor. CH3 0 CH:CHCH2OCCH; HCOCHa isobutyl acetate 2-methylpropyl ethanoate benzyl formate benzyl methanoate Chapter 21 3
Chapter 21 3 Naming Esters • Esters are named as alkyl carboxylates. • Alkyl from the alcohol, carboxylate from the carboxylic acid precursor. isobutyl acetate 2-methylpropyl ethanoate CH3CHCH2OCCH3 CH3 O HCOCH2 O benzyl formate benzyl methanoate =>
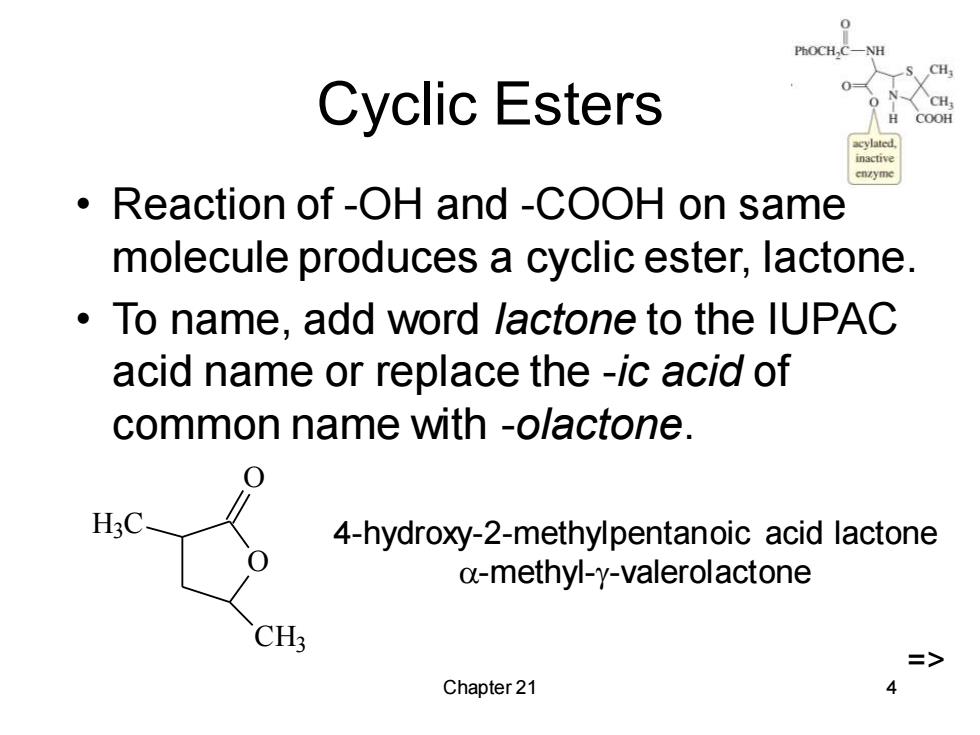
H Cyclic Esters COOH Reaction of-OH and -COOH on same molecule produces a cyclic ester,lactone. To name,add word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. H3C 4-hydroxy-2-methylpentanoic acid lactone a-methyl-y-valerolactone CH3 => Chapter 21 4
Chapter 21 4 Cyclic Esters • Reaction of -OH and -COOH on same molecule produces a cyclic ester, lactone. • To name, add word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. O O H3 C CH3 4-hydroxy-2-methylpentanoic acid lactone -methyl--valerolactone =>
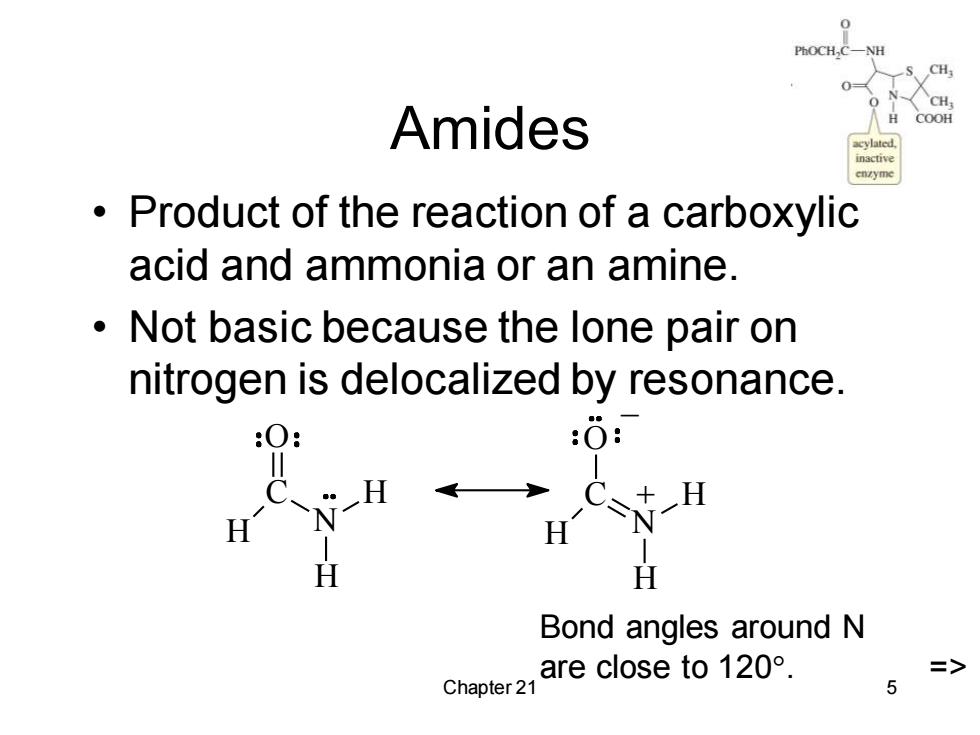
Amides COOH Product of the reaction of a carboxylic acid and ammonia or an amine. Not basic because the lone pair on nitrogen is delocalized by resonance. :0: H H H CN H H Bond angles around N are close to120°. Chapter 21 5
Chapter 21 5 Amides • Product of the reaction of a carboxylic acid and ammonia or an amine. • Not basic because the lone pair on nitrogen is delocalized by resonance. H C O N H H H C O N H H _ + Bond angles around N are close to 120. =>