
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 22 Alpha Substitution and Condensations of Enols and Enolate lons Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,C=O. Step 1:Deprotonation Step 2:Attack on electrophile H enolate ion Chapter 22
Chapter 22 2 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl, C=O. enolate ion =>
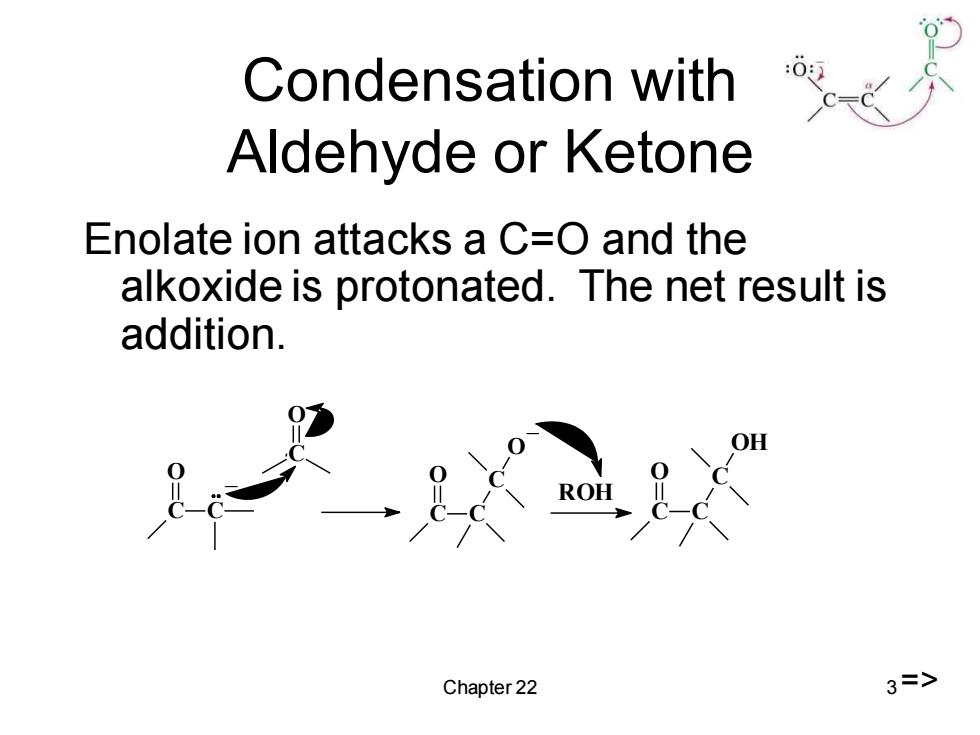
Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated.The net result is addition. ROH Chapter 22 3=>
Chapter 22 3 Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated. The net result is addition. C O C _ C O C O C C O _ ROH C O C C OH =>

Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution. Step 1:Addition of the enolate Step 2:Elimination of the alkoxide C=0 COR- +RO- enolate ester substitution product Chapter 22 4
Chapter 22 4 Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution. =>
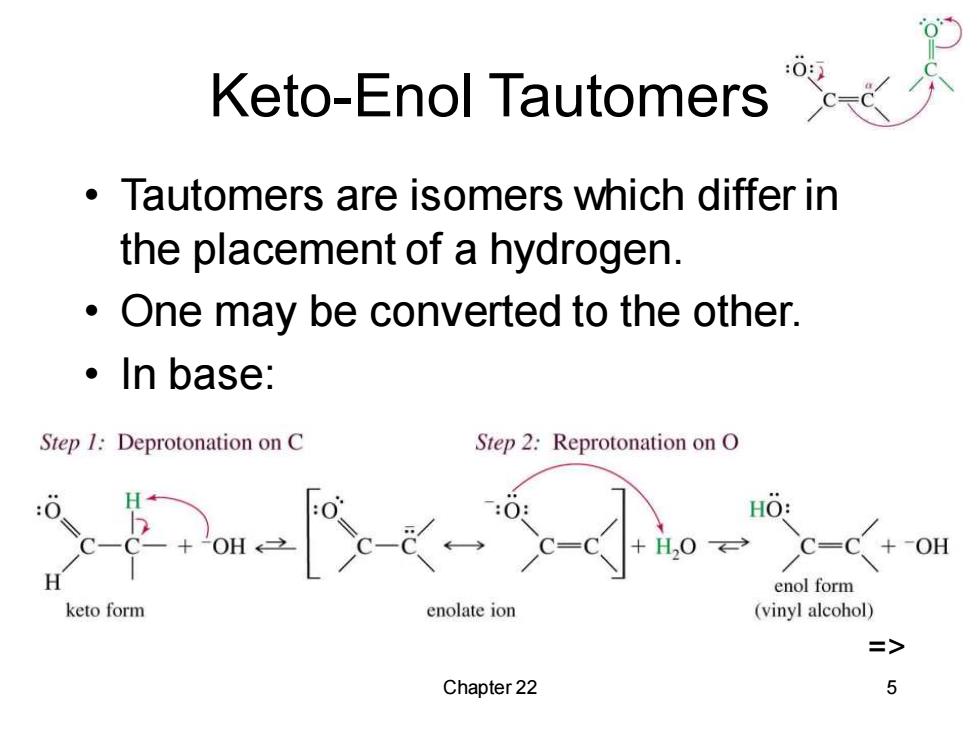
Keto-Enol Tautomers Tautomers are isomers which differ in the placement of a hydrogen. One may be converted to the other. 。In base: Step 1:Deprotonation on C Step 2:Reprotonation on O HO: C=( enol form keto form enolate ion (vinyl alcohol) Chapter 22 5
Chapter 22 5 Keto-Enol Tautomers • Tautomers are isomers which differ in the placement of a hydrogen. • One may be converted to the other. • In base: =>