
Organic Chemistry,7th Editior L.G.Wade,Jr. H Chapter 9 Alkynes Copyright 2010 Pearson Education,Inc
Chapter 9 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Alkynes

Introduction Alkynes contain a triple bond. General formula is CnH2n-2. Two elements of unsaturation for each triple bond. Some reactions resemble the reactions of alkenes,like addition and oxidation. Some reactions are specific to alkynes. Chapter 9 2
Chapter 9 2 Introduction • Alkynes contain a triple bond. • General formula is CnH2n-2 . • Two elements of unsaturation for each triple bond. • Some reactions resemble the reactions of alkenes, like addition and oxidation. • Some reactions are specific to alkynes
Nomenclature:IUPAC Find the longest chain containing the triple bond. Change -ane ending to -yne. Number the chain,starting at the end closest to the triple bond. Give branches or other substituents a number to locate their position. Chapter 9 3
Chapter 9 3 Nomenclature: IUPAC • Find the longest chain containing the triple bond. • Change -ane ending to -yne. • Number the chain, starting at the end closest to the triple bond. • Give branches or other substituents a number to locate their position
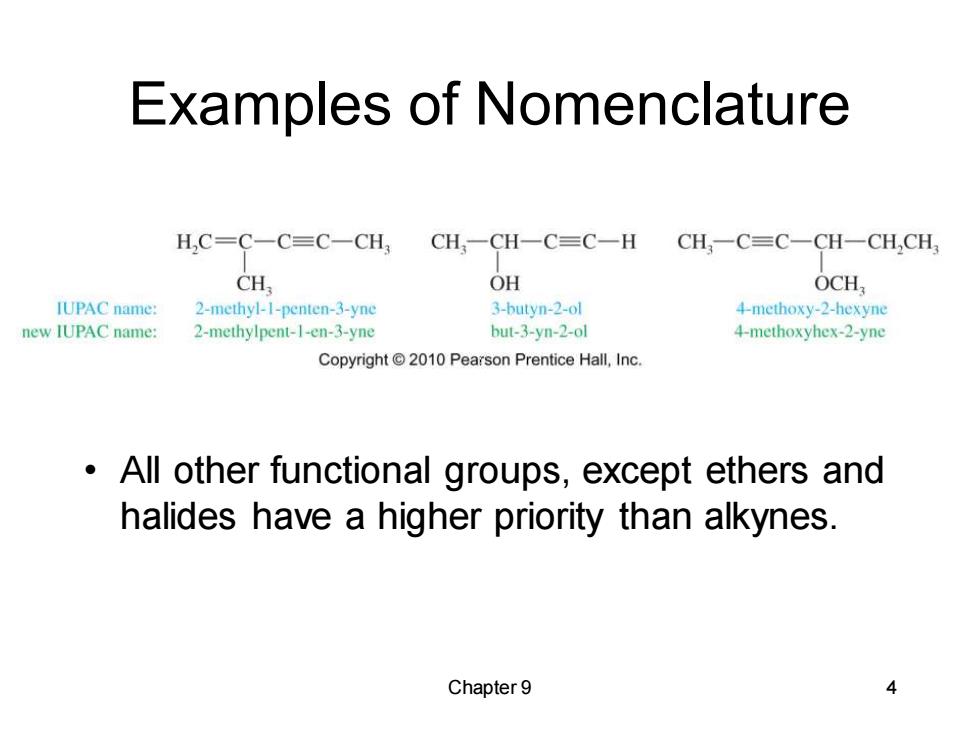
Examples of Nomenclature H,C=C一C=C-CH3CH,一CH一C=C-H CH一C=C-CH一CH,CH CH OH OCH, IUPAC name: 2-methyl-I-penten-3-yne 3-butyn-2-ol 4-methoxy-2-hexyne new IUPAC name: 2-methylpent-1-en-3-yne but-3-yn-2-ol 4-methoxyhex-2-yne Copyright 2010 Pearson Prentice Hall,Inc. All other functional groups,except ethers and halides have a higher priority than alkynes. Chapter 9 4
Chapter 9 4 Examples of Nomenclature • All other functional groups, except ethers and halides have a higher priority than alkynes

Common Names Named as substituted acetylene. CH3-C=CH methylacetylene (terminal alkyne) CH3 CH3 CH3-CH-CH2-C=C-CH-CH3 isobutylisopropylacetylene (internal alkyne) Chapter 9 5
Chapter 9 5 Common Names Named as substituted acetylene. methylacetylene (terminal alkyne) isobutylisopropylacetylene (internal alkyne) CH3 CH CH3 CH2 C C CH CH3 CH3 CH3 C CH