Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 17 Reactions of Aromatic Compounds Copyright 2010 Pearson Education,Inc
Chapter 17 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Reactions of Aromatic Compounds

Electrophilic Aromatic Substitution E H H B-H attack on an electrophile sigma complex substituted Copyright2010 Pearson Prentice Hall,Inc. Although benzene's pi electrons are in a stable aromatic system,they are available to attack a strong electrophile to give a carbocation. This resonance-stabilized carbocation is called a sigma complex because the electrophile is joined to the benzene ring by a new sigma bond. Aromaticity is regained by loss of a proton. Chapter 17 2
Chapter 17 2 Electrophilic Aromatic Substitution ▪ Although benzene’s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give a carbocation. ▪ This resonance-stabilized carbocation is called a sigma complex because the electrophile is joined to the benzene ring by a new sigma bond. ▪ Aromaticity is regained by loss of a proton
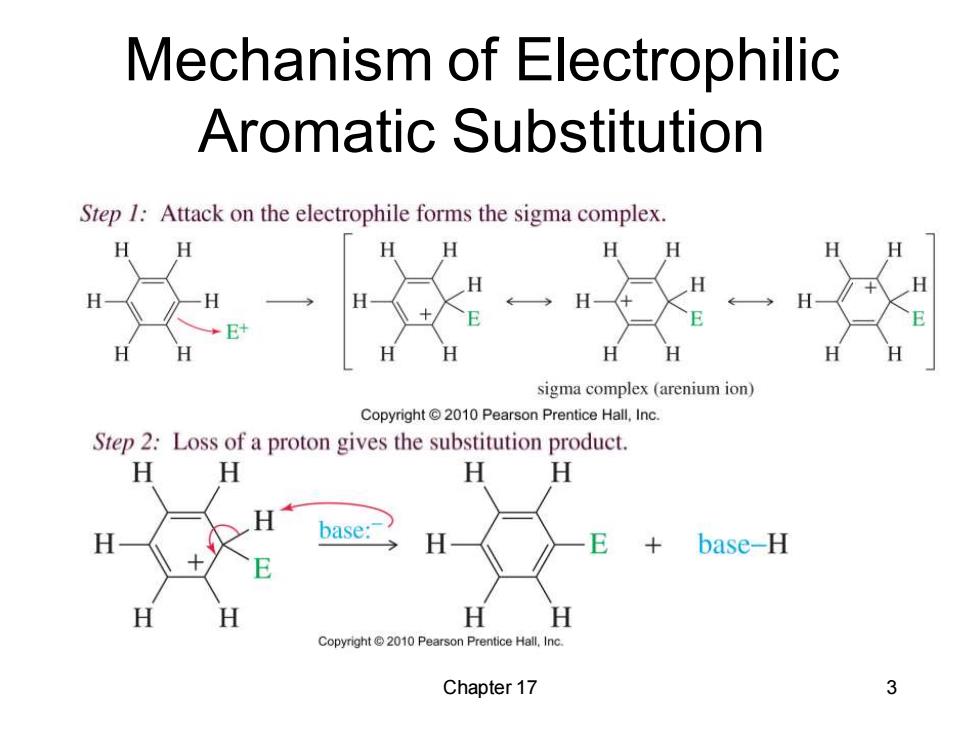
Mechanism of Electrophilic Aromatic Substitution Step 1:Attack on the electrophile forms the sigma complex. H 6 H H E+ H H H sigma complex(arenium ion) Copyright 2010 Pearson Prentice Hall,Inc. Step 2:Loss of a proton gives the substitution product. H H H H H base: base-H H H H H Copyright @2010 Pearson Prentice Hall,Inc. Chapter 17 3
Chapter 17 3 Mechanism of Electrophilic Aromatic Substitution
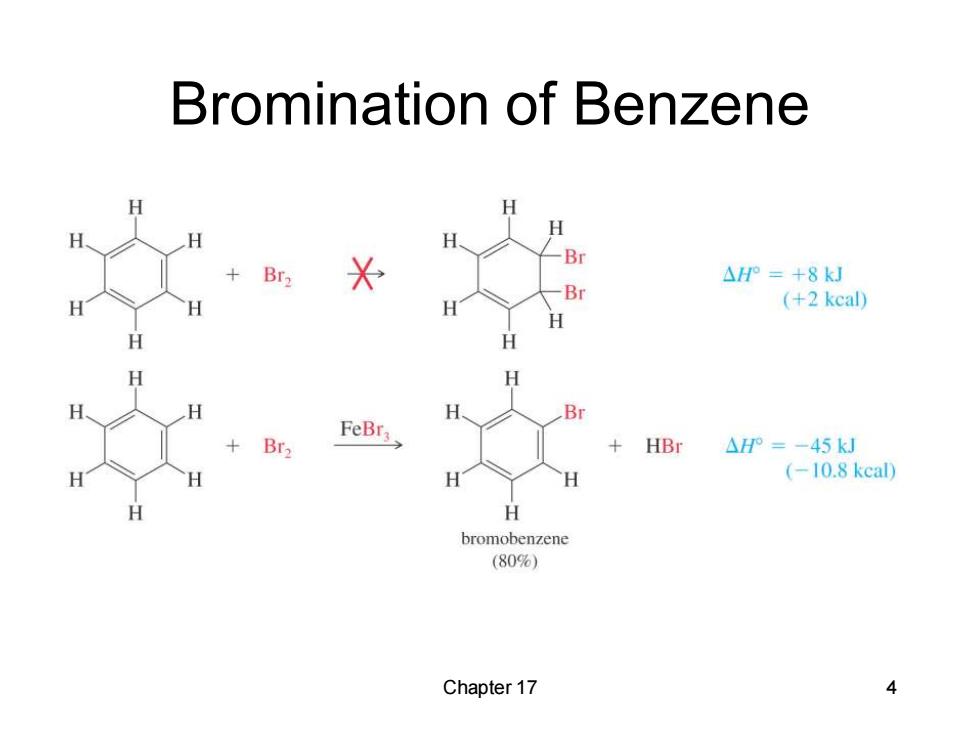
Bromination of Benzene H H H H Br +Br2 △P=+8kJ Br H H (+2 kcal) H H H Br FeBr3> +HBr △HP=-45kJ H H (-10.8kcal) bromobenzene (80%)】 Chapter 17 4
Chapter 17 4 Bromination of Benzene
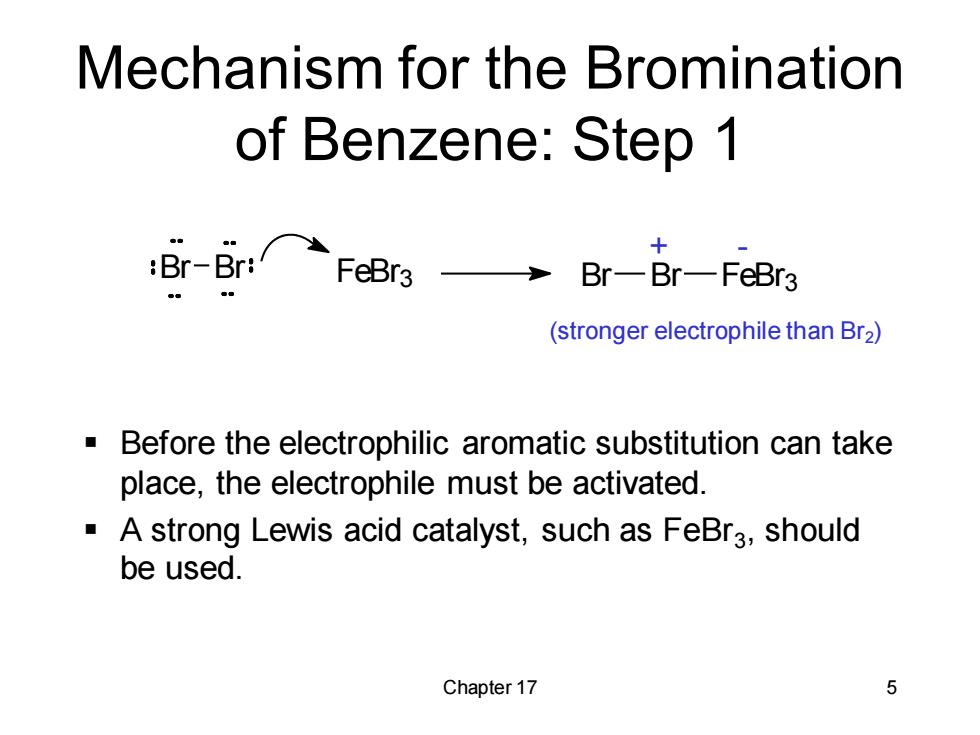
Mechanism for the Bromination of Benzene:Step 1 :Br--Br:FeBra3→ 十 Br-Br-FeBr3 (stronger electrophile than Br2) Before the electrophilic aromatic substitution can take place,the electrophile must be activated. A strong Lewis acid catalyst,such as FeBra,should be used. Chapter 17 5
Chapter 17 5 Mechanism for the Bromination of Benzene: Step 1 ▪ Before the electrophilic aromatic substitution can take place, the electrophile must be activated. ▪ A strong Lewis acid catalyst, such as FeBr3 , should be used. Br Br FeBr3 Br Br FeBr3 + - (stronger electrophile than Br2)