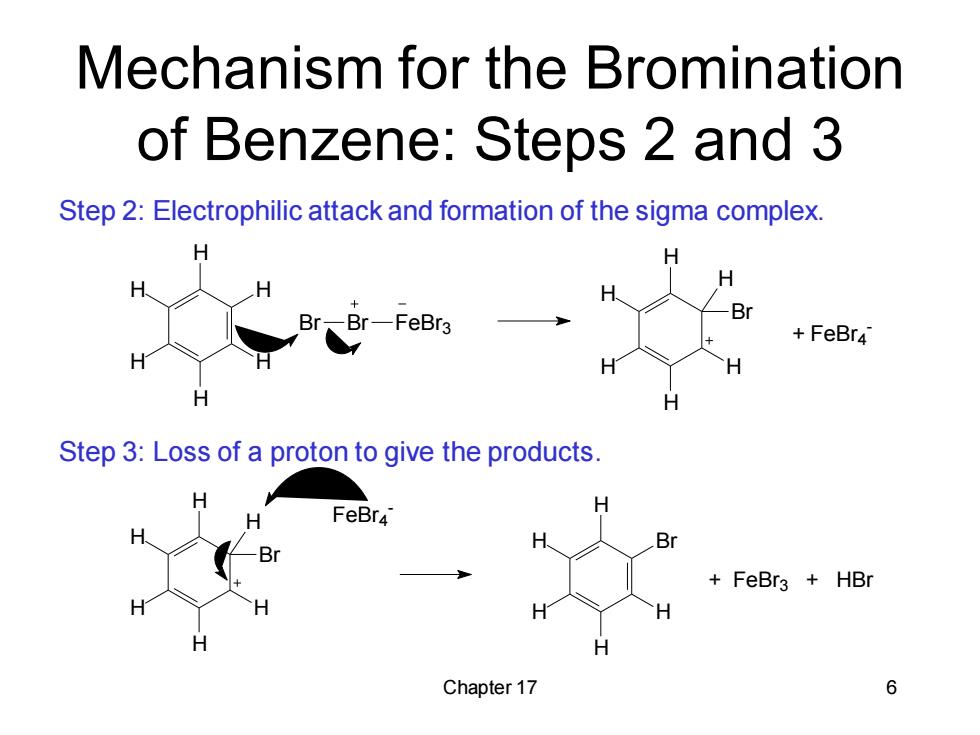
Mechanism for the Bromination of Benzene:Steps 2 and 3 Step 2:Electrophilic attack and formation of the sigma complex. H H -FeBr3 Br FeBr4 H H Step 3:Loss of a proton to give the products. FeBr4 H Br Br FeBr3 +HBr H H H Chapter 17 6
Chapter 17 6 H H H H H H Br Br FeBr3 H H H H H H Br + FeBr4 - H H H H H H Br FeBr4 - Br H H H H H + FeBr3 + HBr Step 2: Electrophilic attack and formation of the sigma complex. Step 3: Loss of a proton to give the products. Mechanism for the Bromination of Benzene: Steps 2 and 3
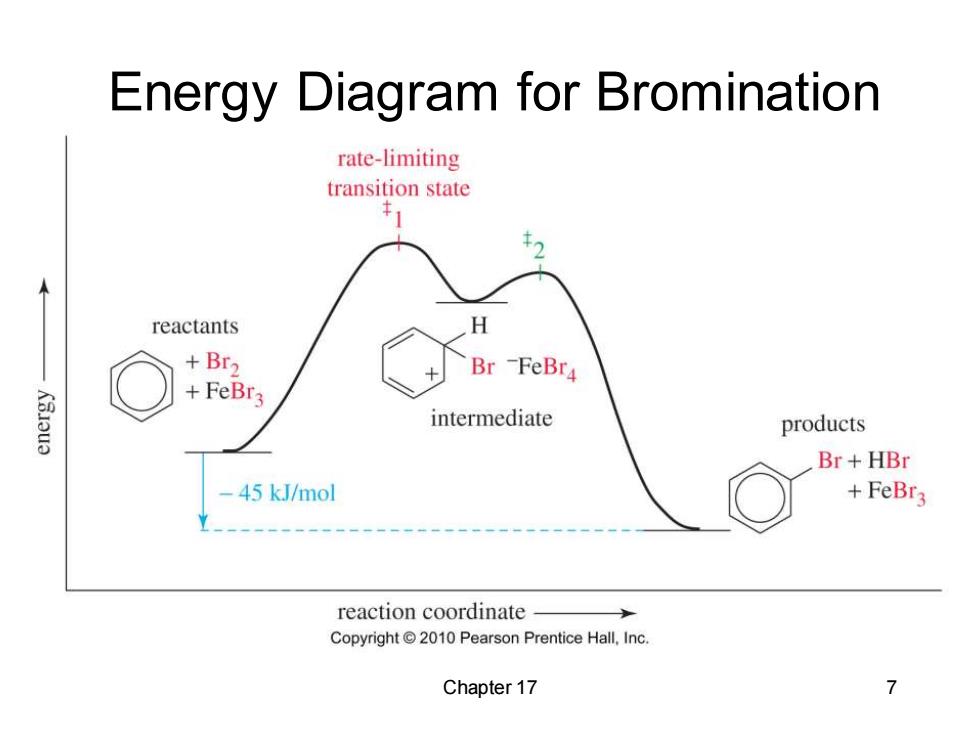
Energy Diagram for Bromination rate-limiting transition state reactants H +Br2 Br -FeBr4 +FeBr3 K3I3u3 intermediate products Br+HBr 45 kJ/mol +FeBr3 reaction coordinate Copyright2010 Pearson Prentice Hall,Inc. Chapter 17 7
Chapter 17 7 Energy Diagram for Bromination

Chlorination and lodination Chlorination is similar to bromination. AICla is most often used as catalyst,but FeCla will also work. lodination requires an acidic oxidizing agent,like nitric acid,to produce iodide cation. H+HNO3 +712I*+NO2 H2O Chapter 17 8
Chapter 17 8 Chlorination and Iodination ▪ Chlorination is similar to bromination. AlCl3 is most often used as catalyst, but FeCl3 will also work. ▪ Iodination requires an acidic oxidizing agent, like nitric acid, to produce iodide cation. H + + HNO3 + ½ I2 I + + NO2 + H2O
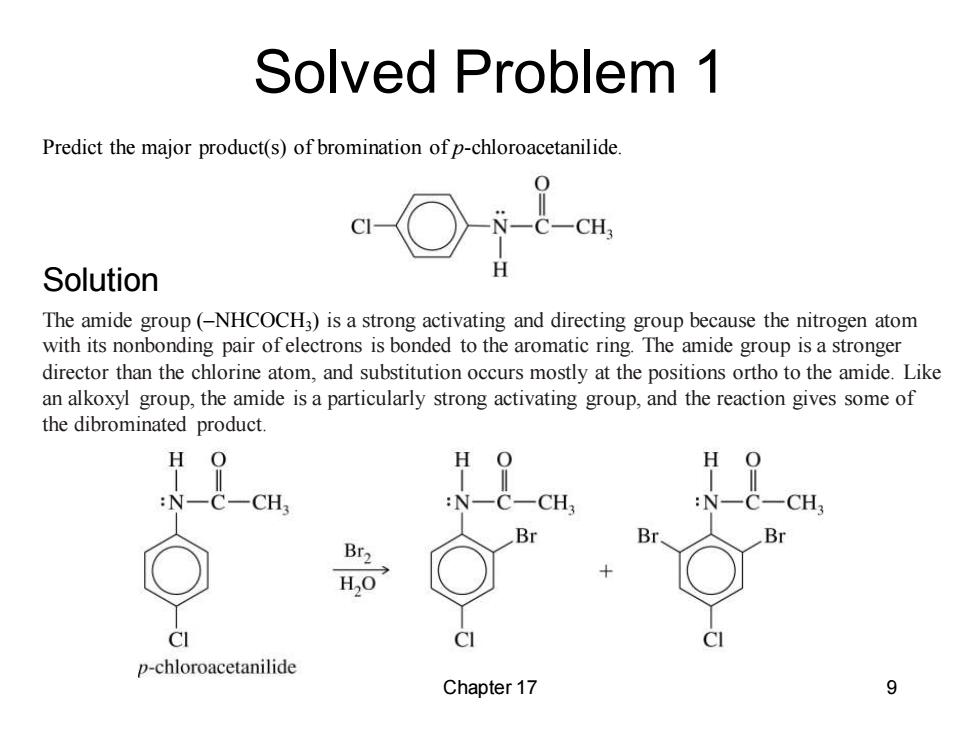
Solved Problem 1 Predict the major product(s)of bromination of p-chloroacetanilide. C-CH Solution The amide group(-NHCOCH3)is a strong activating and directing group because the nitrogen atom with its nonbonding pair ofelectrons is bonded to the aromatic ring.The amide group is a stronger director than the chlorine atom,and substitution occurs mostly at the positions ortho to the amide.Like an alkoxyl group,the amide is a particularly strong activating group,and the reaction gives some of the dibrominated product. H O H :N-C-CH3 :N- CH N C一CH Br2 H2O CI p-chloroacetanilide Chapter 17 9
Chapter 17 9 Predict the major product(s) of bromination of p-chloroacetanilide. The amide group (–NHCOCH3 ) is a strong activating and directing group because the nitrogen atom with its nonbonding pair of electrons is bonded to the aromatic ring. The amide group is a stronger director than the chlorine atom, and substitution occurs mostly at the positions ortho to the amide. Like an alkoxyl group, the amide is a particularly strong activating group, and the reaction gives some of the dibrominated product. Solved Problem 1 Solution
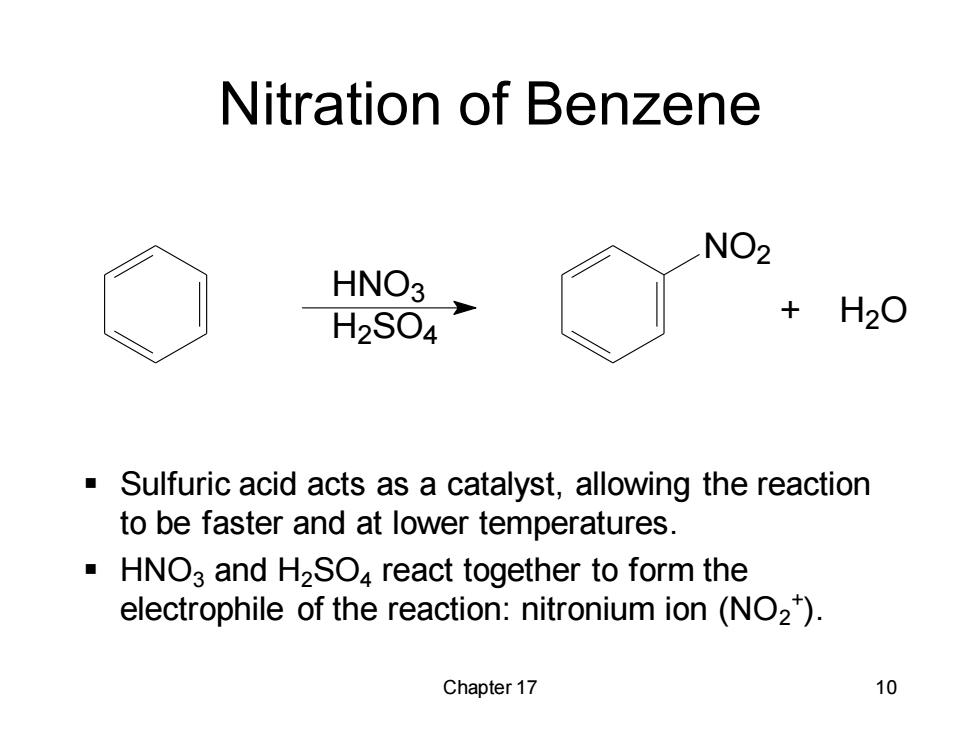
Nitration of Benzene NO2 HNO3 H2S04 H2O Sulfuric acid acts as a catalyst,allowing the reaction to be faster and at lower temperatures. HNO3 and H2SO4 react together to form the electrophile of the reaction:nitronium ion(NO2). Chapter 17 10
Chapter 17 10 Nitration of Benzene ▪ Sulfuric acid acts as a catalyst, allowing the reaction to be faster and at lower temperatures. ▪ HNO3 and H2SO4 react together to form the electrophile of the reaction: nitronium ion (NO2 + ). HNO3 H2 SO4 NO2 + H2 O