
R Organic Chemistry,7th Edition H O—R L.G.Wade,Jr. H-C-Mg-X H R—O④ R Chapter 14 Ethers,Epoxides,and Sulfides Copyright 2010 Pearson Education,Inc
Chapter 14 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Ethers, Epoxides, and Sulfides
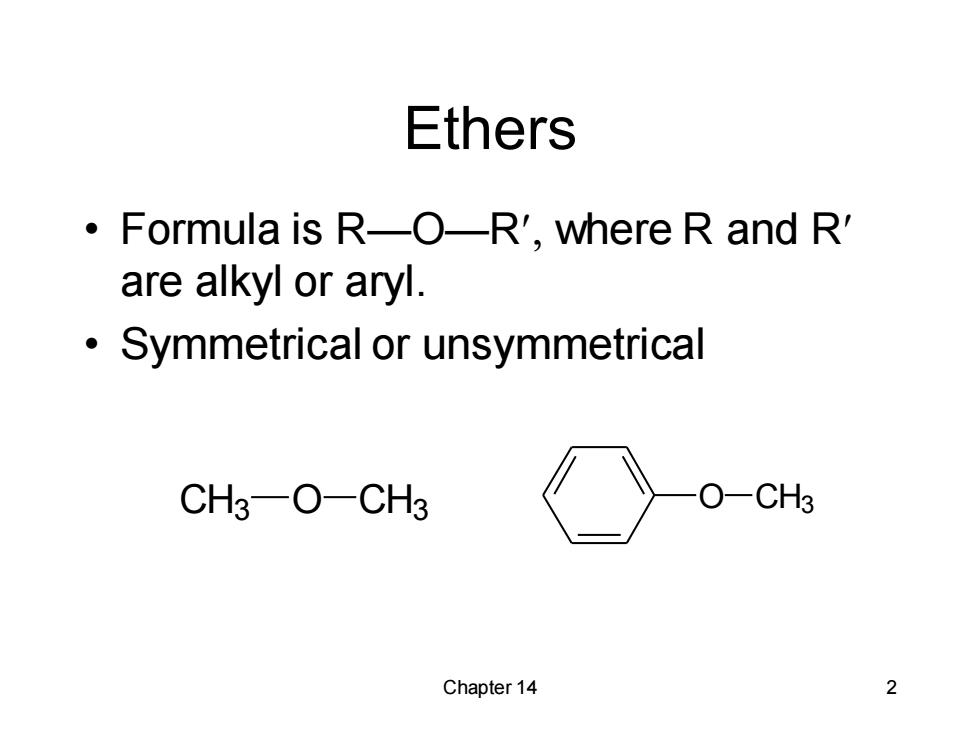
Ethers Formula is R-O-R',where R and R' are alkyl or aryl. Symmetrical or unsymmetrical CH3-O-CH3 O-CH3 Chapter 14 2
Chapter 14 2 Ethers • Formula is R—O—R where R and R are alkyl or aryl. • Symmetrical or unsymmetrical CH3 O CH3 O CH3
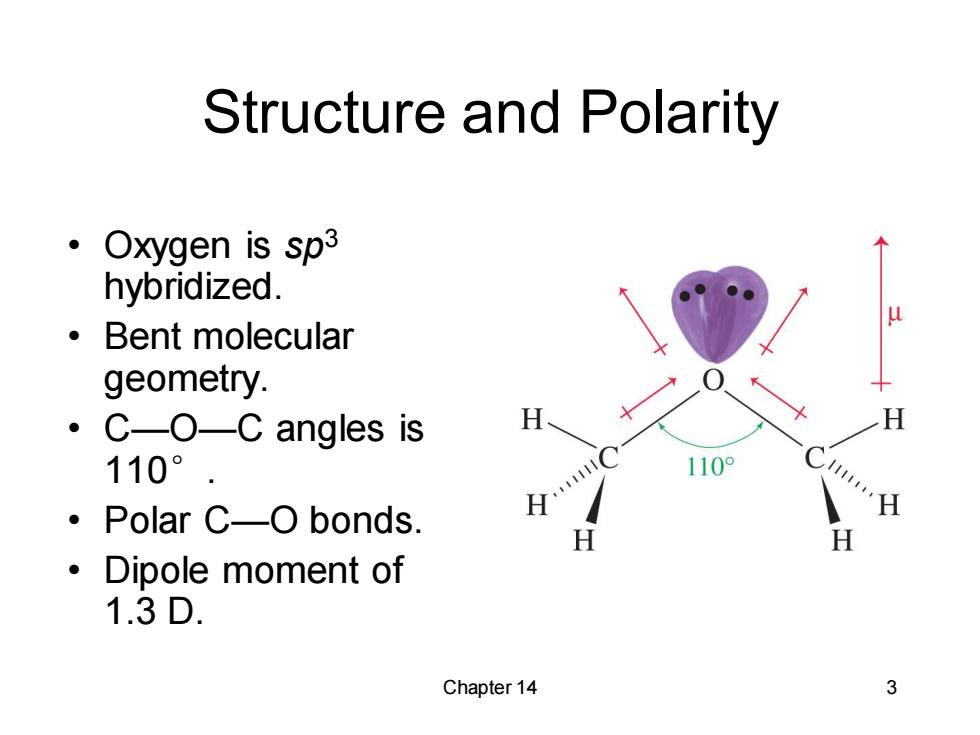
Structure and Polarity 。( Oxygen is sp3 hybridized. 。Bent molecular geometry. 。 C-O-C angles is 110° 。Polar C-O bonds. H ·Dipole moment of 1.3D. Chapter 14 3
Chapter 14 3 Structure and Polarity • Oxygen is sp3 hybridized. • Bent molecular geometry. • C—O—C angles is 110°. • Polar C—O bonds. • Dipole moment of 1.3 D
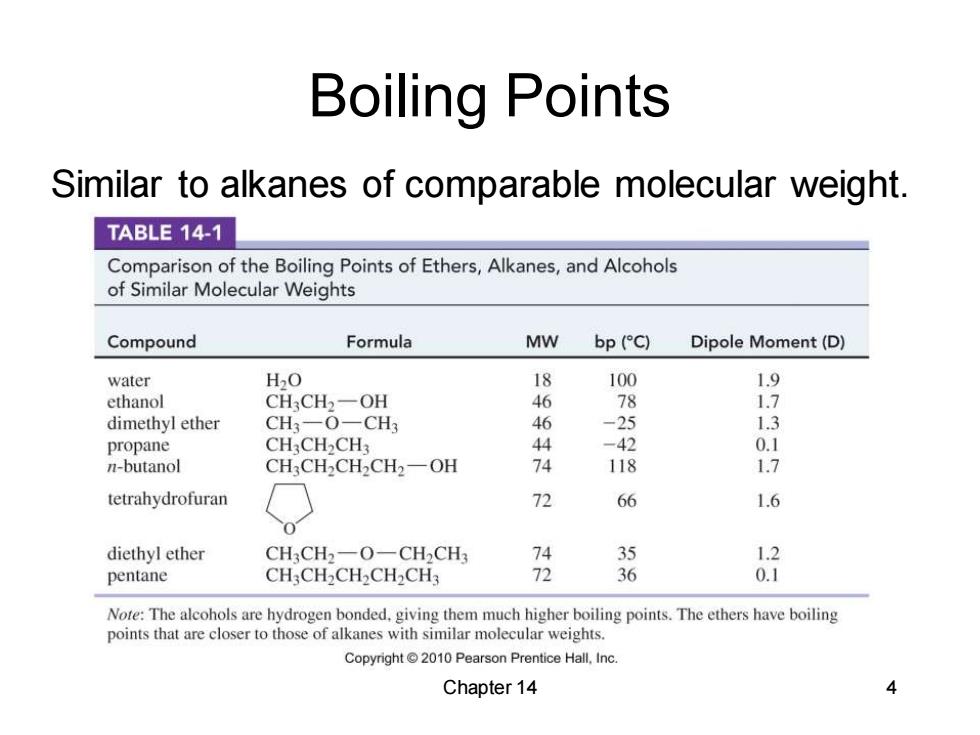
Boiling Points Similar to alkanes of comparable molecular weight. TABLE 14-1 Comparison of the Boiling Points of Ethers,Alkanes,and Alcohols of Similar Molecular Weights Compound Formula MW bp (C) Dipole Moment(D) water H2O 18 100 ethanol CH3CH2一OH 46 78 dimethyl ether CH3-O一CH3 46 -25 1.3 propane CH3CH2CH3 44 -42 n-butanol CH3CH2CH2CH2-OH 74 118 1.7 tetrahydrofuran 72 66 1.6 diethyl ether CH3CH2一O-CH2CH 35 1.2 pentane CH3CH2CH2CH2CH3 72 36 0.1 Note:The alcohols are hydrogen bonded.giving them much higher boiling points.The ethers have boiling points that are closer to those of alkanes with similar molecular weights. Copyright 2010 Pearson Prentice Hall,Inc Chapter 14
Chapter 14 4 Boiling Points Similar to alkanes of comparable molecular weight
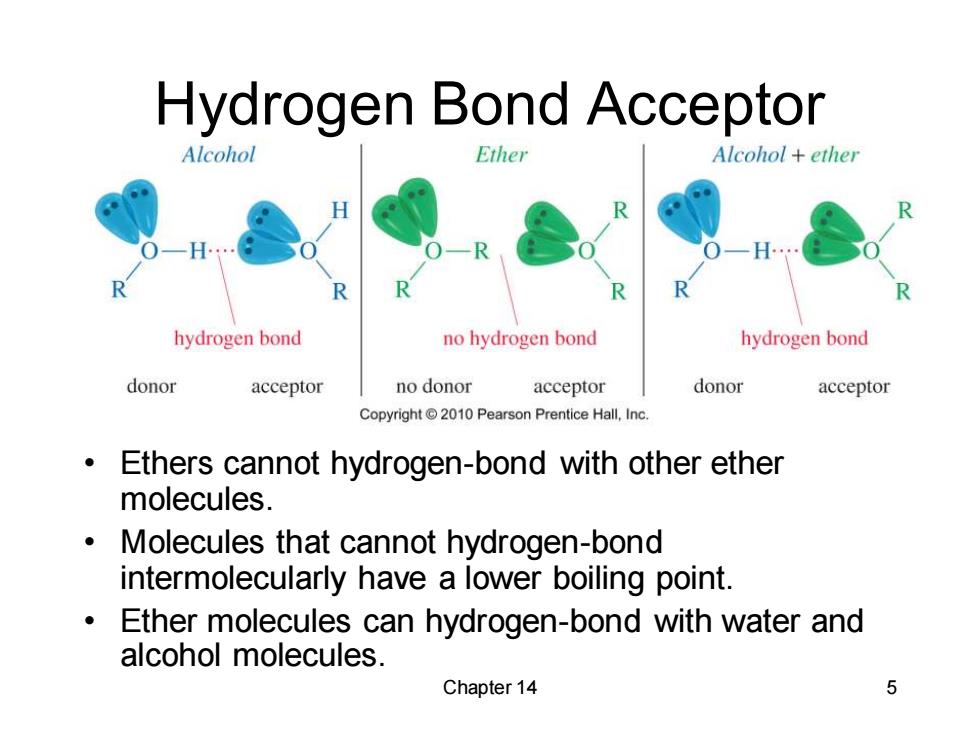
Hydrogen Bond Acceptor Alcohol Ether Alcohol ether hydrogen bond no hydrogen bond hydrogen bond donor acceptor no donor acceptor donor acceptor Copyright 2010 Pearson Prentice Hall,Inc. Ethers cannot hydrogen-bond with other ether molecules. Molecules that cannot hydrogen-bond intermolecularly have a lower boiling point. Ether molecules can hydrogen-bond with water and alcohol molecules. Chapter 14 5
Chapter 14 5 Hydrogen Bond Acceptor • Ethers cannot hydrogen-bond with other ether molecules. • Molecules that cannot hydrogen-bond intermolecularly have a lower boiling point. • Ether molecules can hydrogen-bond with water and alcohol molecules