Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds Copyright 2010 Pearson Education,Inc
Chapter 22 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Condensations and Alpha Substitutions of Carbonyl Compounds
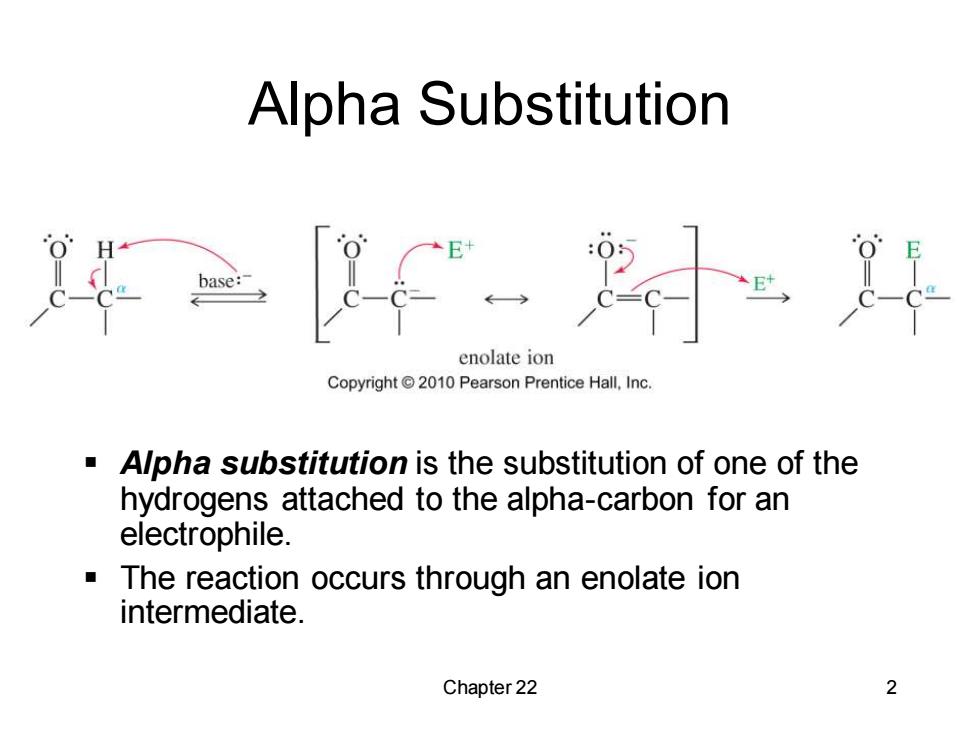
Alpha Substitution enolate ion Copyright 2010 Pearson Prentice Hall,Inc. Alpha substitution is the substitution of one of the hydrogens attached to the alpha-carbon for an electrophile. The reaction occurs through an enolate ion intermediate. Chapter 22 2
Chapter 22 2 Alpha Substitution ▪ Alpha substitution is the substitution of one of the hydrogens attached to the alpha-carbon for an electrophile. ▪ The reaction occurs through an enolate ion intermediate
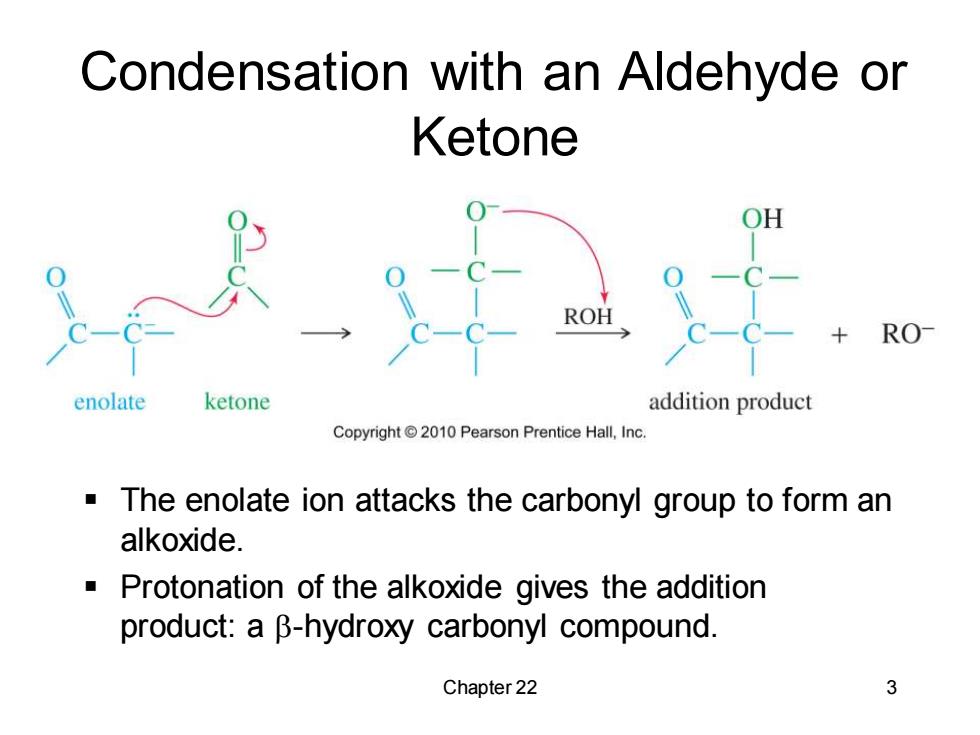
Condensation with an Aldehyde or Ketone OH ROH RO enolate ketone addition product Copyright 2010 Pearson Prentice Hall,Inc The enolate ion attacks the carbonyl group to form an alkoxide. Protonation of the alkoxide gives the addition product:a B-hydroxy carbonyl compound. Chapter 22 3
Chapter 22 3 Condensation with an Aldehyde or Ketone ▪ The enolate ion attacks the carbonyl group to form an alkoxide. ▪ Protonation of the alkoxide gives the addition product: a b-hydroxy carbonyl compound
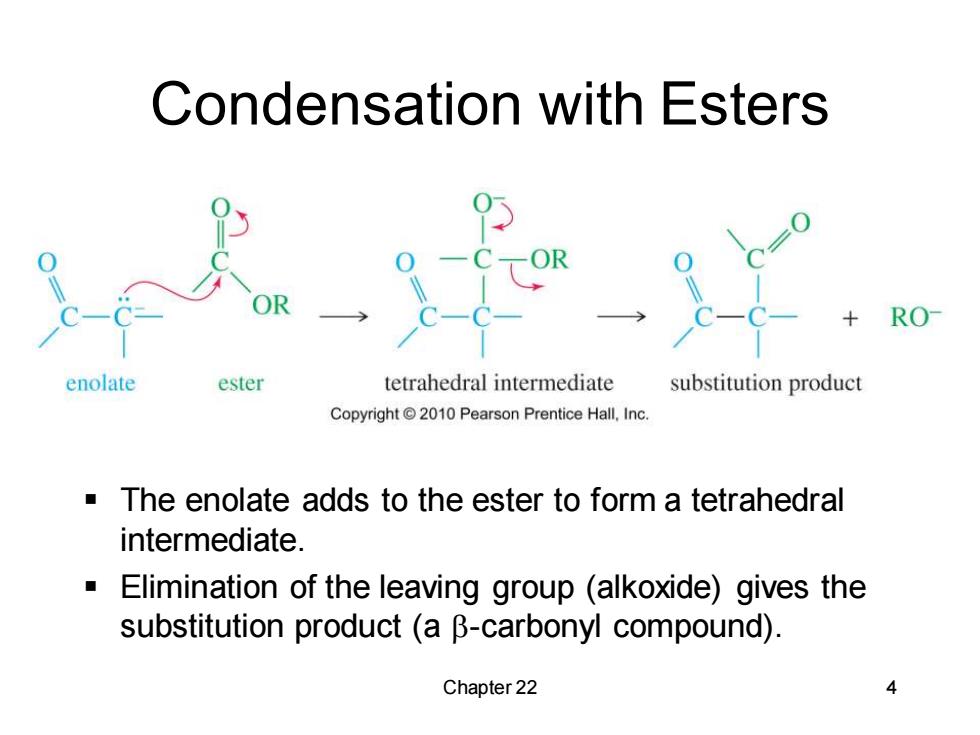
Condensation with Esters RO enolate ester tetrahedral intermediate substitution product Copyright 2010 Pearson Prentice Hall.,Inc. The enolate adds to the ester to form a tetrahedral intermediate. Elimination of the leaving group (alkoxide)gives the substitution product (a B-carbonyl compound). Chapter 22 4
Chapter 22 4 Condensation with Esters ▪ The enolate adds to the ester to form a tetrahedral intermediate. ▪ Elimination of the leaving group (alkoxide) gives the substitution product (a b-carbonyl compound)
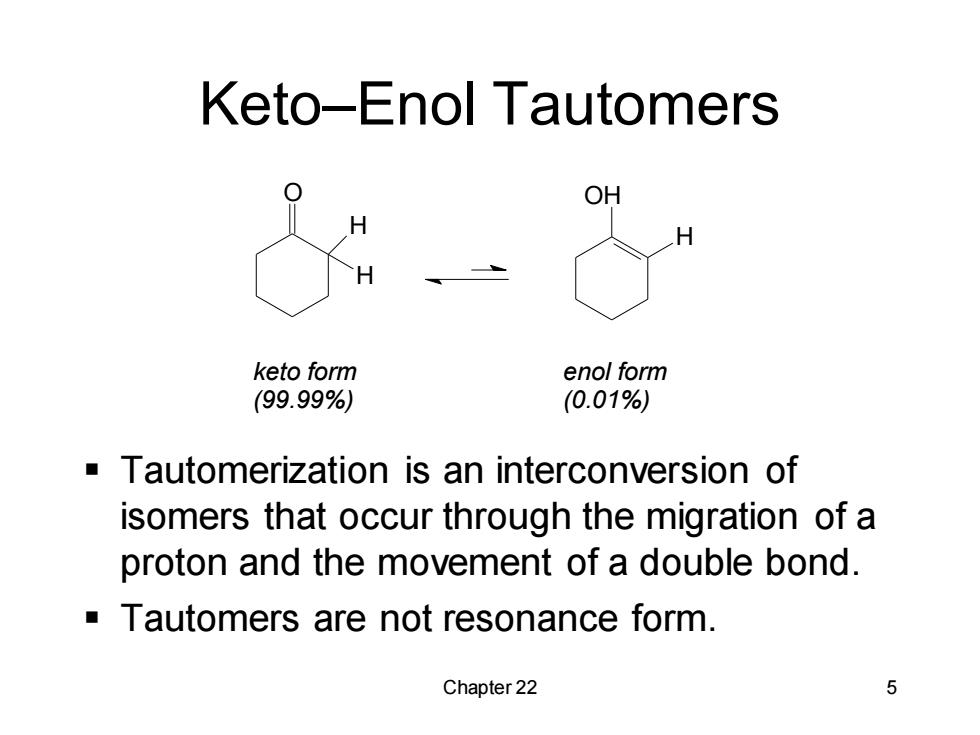
Keto-Enol Tautomers keto form enol form (99.99%) (0.01%) Tautomerization is an interconversion of isomers that occur through the migration of a proton and the movement of a double bond. Tautomers are not resonance form. Chapter 22 5
Chapter 22 5 Keto–Enol Tautomers O H H OH H keto form (99.99%) enol form (0.01%) ▪ Tautomerization is an interconversion of isomers that occur through the migration of a proton and the movement of a double bond. ▪ Tautomers are not resonance form