
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 8 Reactions of Alkenes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©20o3,Prentice Hall
Chapter 8 Reactions of Alkenes Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
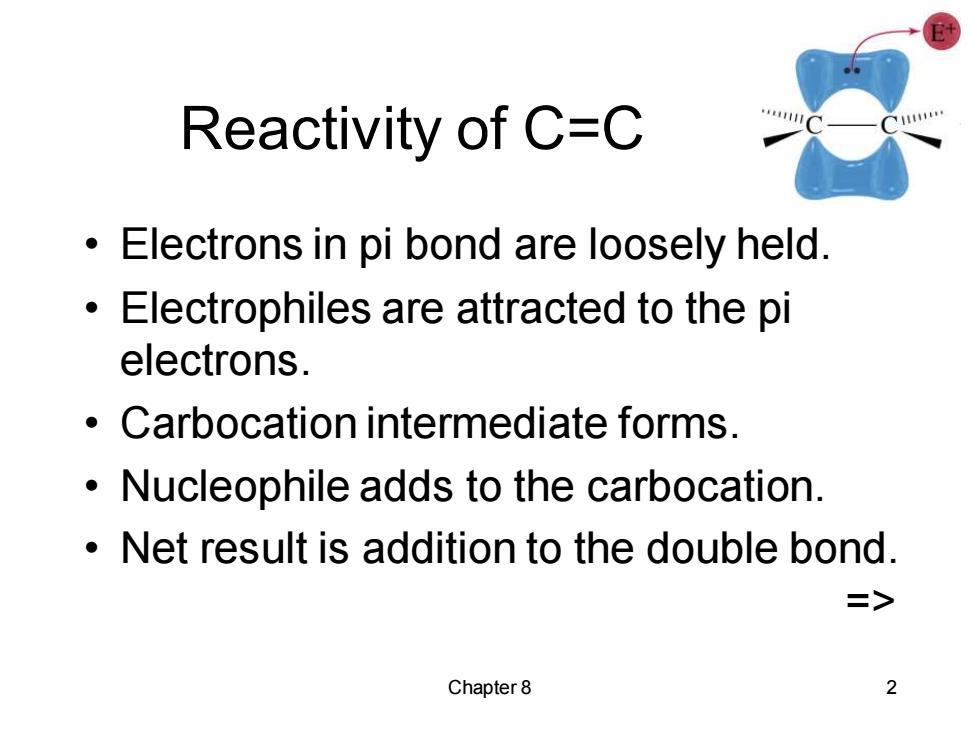
Reactivity of C=C Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. Carbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the double bond. 二> Chapter8 2
Chapter 8 2 Reactivity of C=C • Electrons in pi bond are loosely held. • Electrophiles are attracted to the pi electrons. • Carbocation intermediate forms. • Nucleophile adds to the carbocation. • Net result is addition to the double bond. =>
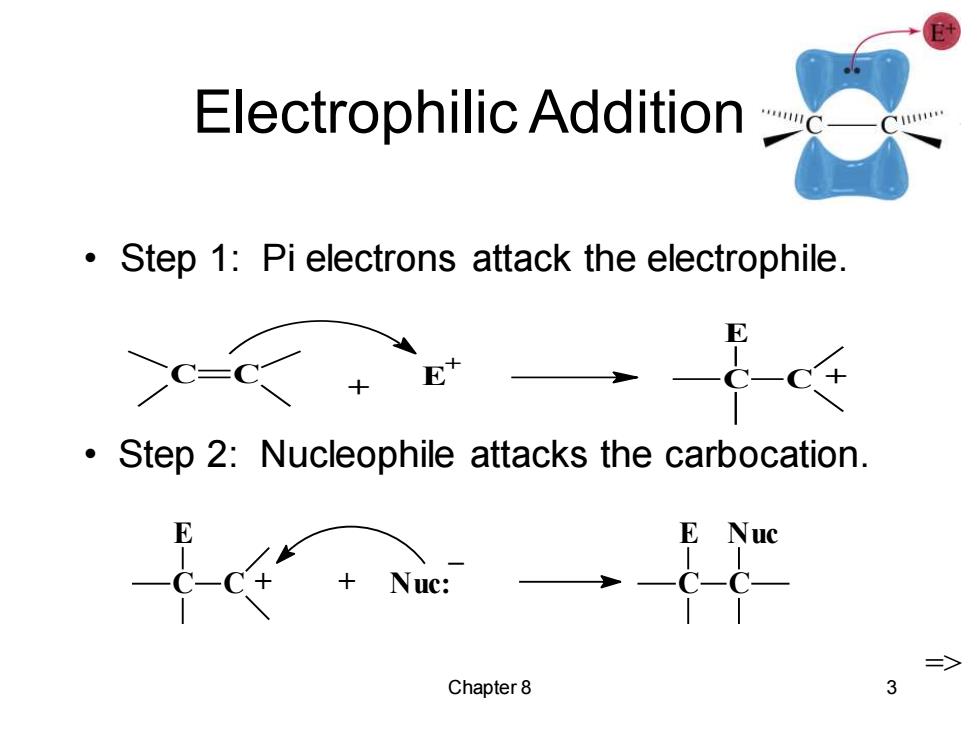
Electrophilic Addition Step 1:Pi electrons attack the electrophile. C-C Step 2:Nucleophile attacks the carbocation. E Nuc +Nuc: => Chapter 8 3
Chapter 8 3 Electrophilic Addition • Step 1: Pi electrons attack the electrophile. C C + E + C E C + C E C + + Nuc: _ C E C Nuc => • Step 2: Nucleophile attacks the carbocation
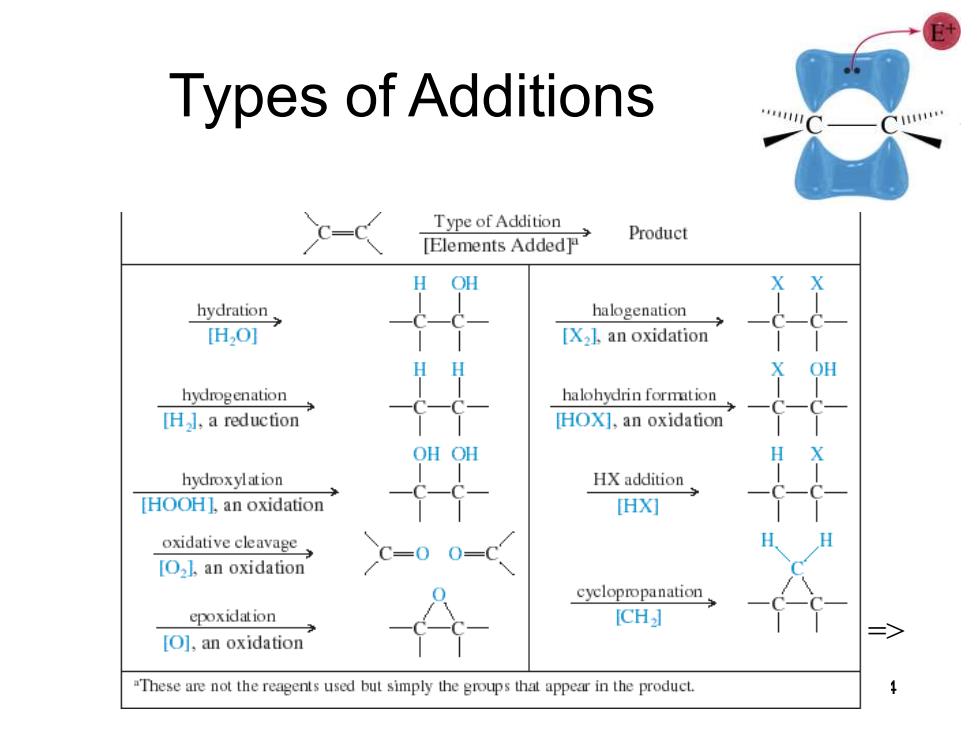
Types of Additions Type of Addition [Elements Added Product H OH hydration halogenation [HO] [X2],an oxidation OH hydrogenation halohydrin formtion [H],a reduction [HOX],an oxidation OH OH hydroxylation HX addition [HOOH],an oxidation HX灯 oxidative cleavage C=0 [O2l.an oxidation cyclopropanation epoxidation ICH] [O],an oxidation "These are not the reagents used but simply the groups that appear in the product
Chapter 8 4 Types of Additions =>
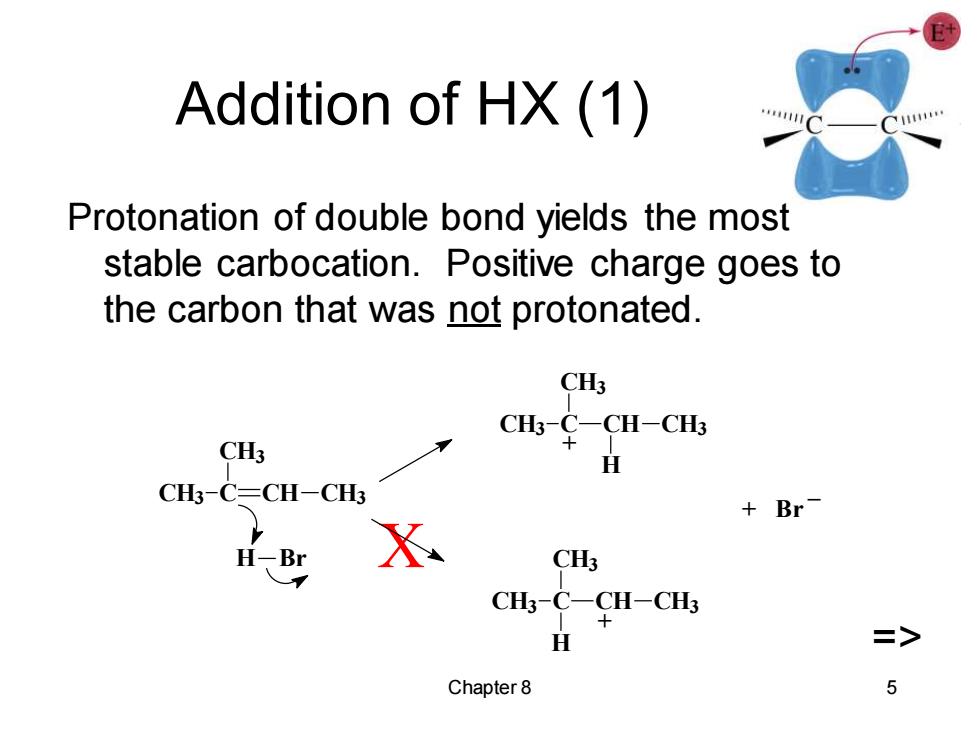
Addition of HX(1) Protonation of double bond yields the most stable carbocation.Positive charge goes to the carbon that was not protonated. CH3 CHs¥一CH-CH CH3 H CH3-C=CH-CH3 +Br H-Br CH3 CH3-C-CH-CH3 H > Chapter 8 5
Chapter 8 5 Addition of HX (1) Protonation of double bond yields the most stable carbocation. Positive charge goes to the carbon that was not protonated. X => + Br _ + + CH3 C CH3 CH CH3 H CH3 C CH3 CH CH3 H H Br CH3 C CH3 CH CH3