
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 7 Structure and Synthesis of Alkenes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 7 Structure and Synthesis of Alkenes Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
Functional Group Pi bond is the functional group. More reactive than sigma bond. Bond dissociation energies: >C=C BDE 146 kcal/mol >C-C BDE -83 kcal/mol >Pi bond 63 kcal/mol => Chapter 7 2
Chapter 7 2 Functional Group • Pi bond is the functional group. • More reactive than sigma bond. • Bond dissociation energies: ➢C=C BDE 146 kcal/mol ➢C-C BDE -83 kcal/mol ➢Pi bond 63 kcal/mol =>
Orbital Description Sigma bonds around C are sp2 hybridized. Angles are approximately 120 degrees. No nonbonding electrons. Molecule is planar around the double bond. Pi bond is formed by the sideways overlap of parallel p orbitals perpendicular to the plane of the molecule. => Chapter 7 3
Chapter 7 3 Orbital Description • Sigma bonds around C are sp2 hybridized. • Angles are approximately 120 degrees. • No nonbonding electrons. • Molecule is planar around the double bond. • Pi bond is formed by the sideways overlap of parallel p orbitals perpendicular to the plane of the molecule. =>
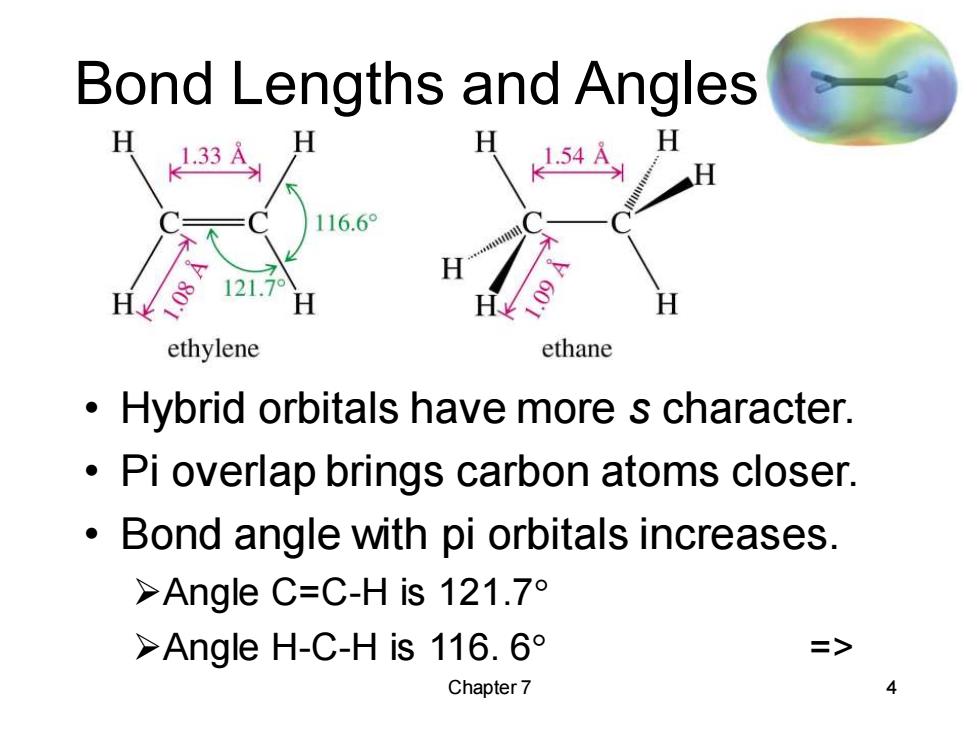
Bond Lengths and Angles 1.33A H 1.54A H 116.6° 121.7° ethylene ethane Hybrid orbitals have more s character. Pi overlap brings carbon atoms closer. Bond angle with pi orbitals increases. >Angle C=C-His121.7° >Angle H-C-His116.6° Chapter 7 4
Chapter 7 4 Bond Lengths and Angles • Hybrid orbitals have more s character. • Pi overlap brings carbon atoms closer. • Bond angle with pi orbitals increases. ➢Angle C=C-H is 121.7 ➢Angle H-C-H is 116. 6 =>
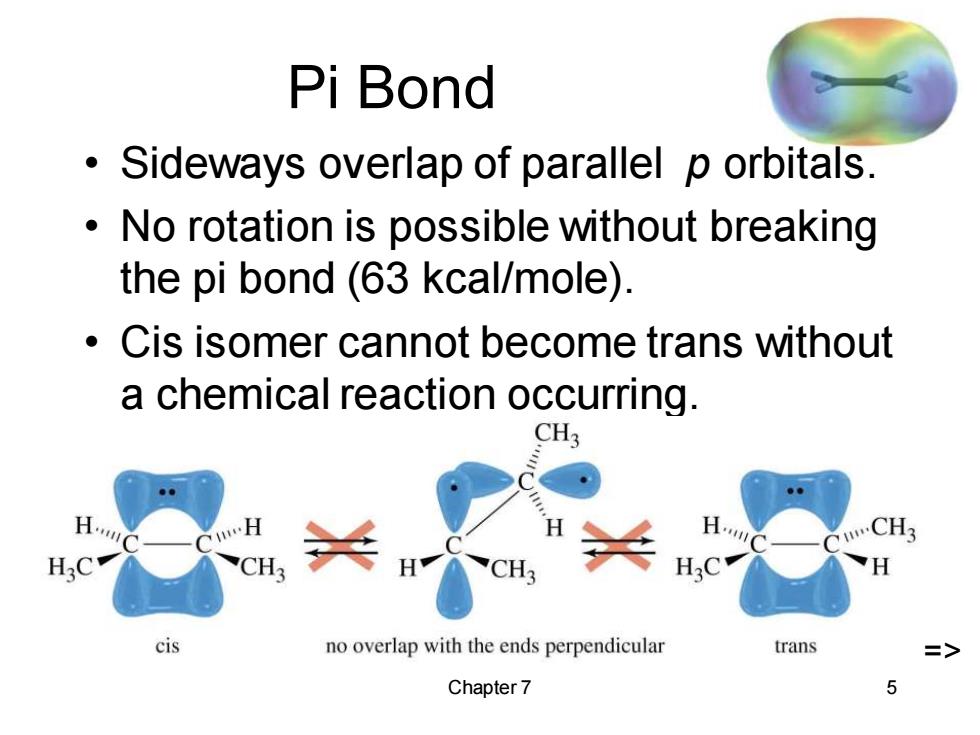
Pi Bond Sideways overlap of parallel p orbitals. No rotation is possible without breaking the pi bond (63 kcal/mole). Cis isomer cannot become trans without a chemical reaction occurring. CH3 CH3 CH cis no overlap with the ends perpendicular trans Chapter 7 5
Chapter 7 5 Pi Bond • Sideways overlap of parallel p orbitals. • No rotation is possible without breaking the pi bond (63 kcal/mole). • Cis isomer cannot become trans without a chemical reaction occurring. =>