
MO Rules for Benzene Six overlapping p orbitals must form six molecular orbitals. Three will be bonding,three antibonding Lowest energy MO will have all bonding interactions,no nodes. As energy of MO increases,the number of nodes increases. Chapter 16 11
Chapter 16 11 MO Rules for Benzene • Six overlapping p orbitals must form six molecular orbitals. • Three will be bonding, three antibonding. • Lowest energy MO will have all bonding interactions, no nodes. • As energy of MO increases, the number of nodes increases
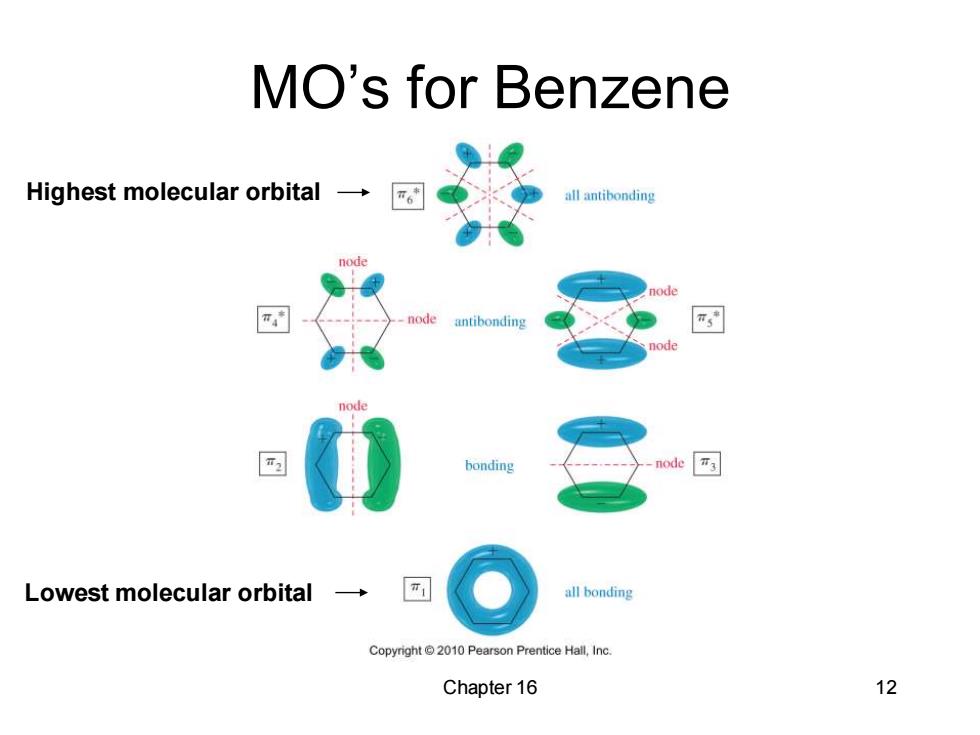
MO's for Benzene Highest molecular orbital → “6 all antibonding node antibonding node bonding node 3 Lowest molecular orbital → all bonding Copyright 2010 Pearson Prentice Hall,Inc. Chapter 16 12
Chapter 16 12 MO’s for Benzene Lowest molecular orbital Highest molecular orbital

First MO of Benzene ·The first MO of all bonding benzene is entirely bonding with no nodes. ·It has very low energy because it has six bonding interactions and the electrons are delocalized over all Copyright2010 Pearson Prentce Hall,Ine six carbon atoms. Chapter 16 13
Chapter 16 13 First MO of Benzene • The first MO of benzene is entirely bonding with no nodes. • It has very low energy because it has six bonding interactions and the electrons are delocalized over all six carbon atoms
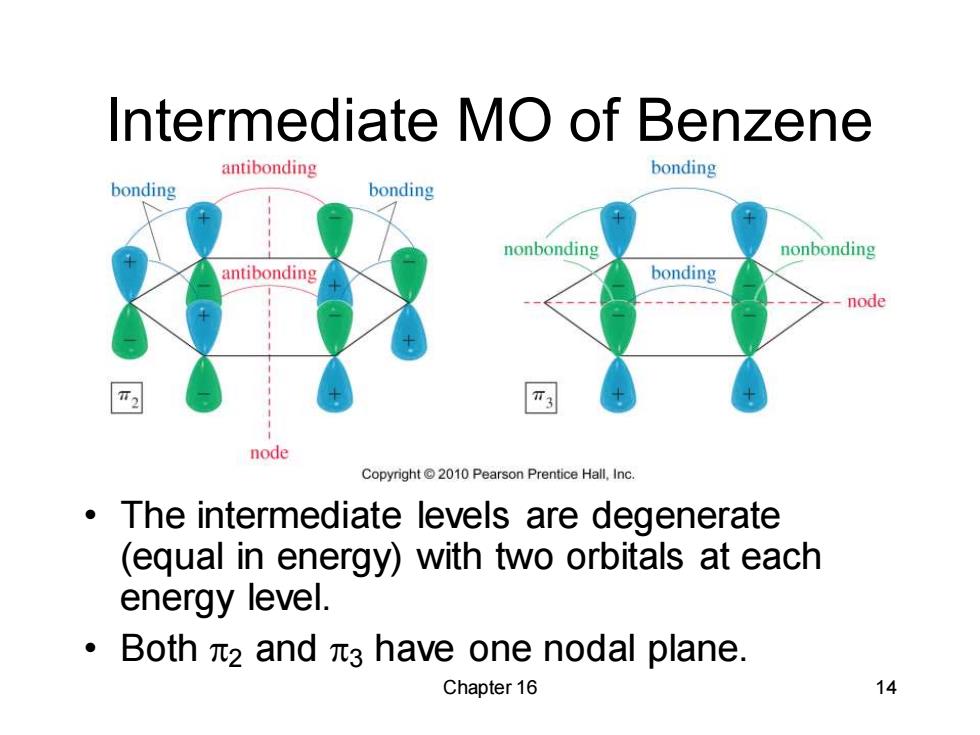
Intermediate MO of Benzene antibonding bonding bonding bonding nonbonding nonbonding antibonding bonding node node Copyright2010 Pearson Prentice Hall.Inc. The intermediate levels are degenerate (equal in energy)with two orbitals at each energy level. ·Bothπ2and元3 have one nodal plane. Chapter 16 14
Chapter 16 14 Intermediate MO of Benzene • The intermediate levels are degenerate (equal in energy) with two orbitals at each energy level. • Both 2 and 3 have one nodal plane
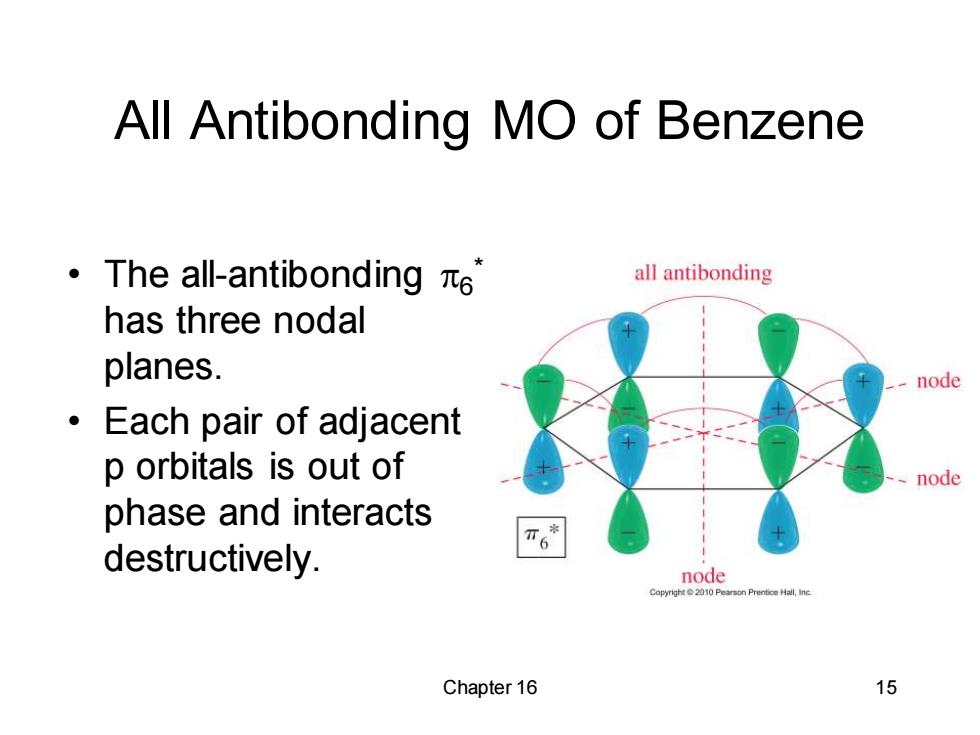
All Antibonding MO of Benzene ·The al-antibonding ie all antibonding has three nodal planes. node ·Each pair of adjacent p orbitals is out of node phase and interacts destructively. node Chapter 16 15
Chapter 16 15 All Antibonding MO of Benzene • The all-antibonding 6 * has three nodal planes. • Each pair of adjacent p orbitals is out of phase and interacts destructively