
Glycerides Fatty acid esters of the triol glycerol Tryglycerides are the most common glycerides and they are used for long-term energy storage in plants and animals. Fats Solid at room temperature. Most are derived from mammals. ·Oils Liquid at room temperature. Most are derived from plants or cold-blooded animals. Chapter 25 6
Chapter 25 6 Glycerides ▪ Fatty acid esters of the triol glycerol. ▪ Tryglycerides are the most common glycerides and they are used for long-term energy storage in plants and animals. ▪ Fats ▪ Solid at room temperature. ▪ Most are derived from mammals. ▪ Oils ▪ Liquid at room temperature. ▪ Most are derived from plants or cold-blooded animals
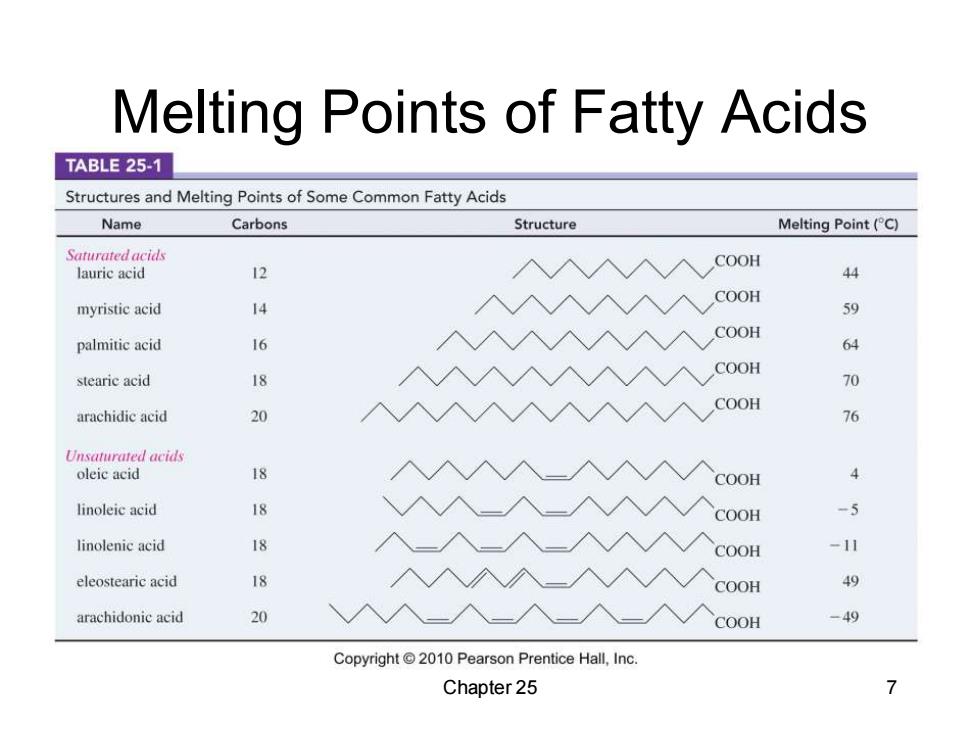
Melting Points of Fatty Acids TABLE 25-1 Structures and Melting Points of Some Common Fatty Acids Name Carbons Structure Melting Point(C) Saturated acids COOH lauric acid 12 44 COOH myristic acid 4 59 COOH palmitic acid 16 64 COOH stearie acid 18 70 arachidic acid 20 COOH 76 Unsaturated acids oleic acid 18 COOH 4 linoleic acid 18 COOH -5 linolenic acid 18 COOH -11 eleostearic acid 18 COOH 49 arachidonic acid 20 COOH -49 Copyright 2010 Pearson Prentice Hall,Inc. Chapter 25 7
Melting Points of Fatty Acids Chapter 25 7

Melting Points COOH COOH stearic acid,mp 70C oleic acid,mp 4C Copyright2010 Pearson Prentice Hall,Inc. The cis double bond in oleic acid lowers the melting point by66°C. A cis double bond bends the molecule,so it cannot pack efficiently. -A trans double bond has less effect. Chapter 25 8
Chapter 25 8 Melting Points ▪ The cis double bond in oleic acid lowers the melting point by 66°C. ▪ A cis double bond bends the molecule, so it cannot pack efficiently. ▪ A trans double bond has less effect

Fats and Oils CH-0 CH一O CH2-0 tristearin,mp 72C 0 CH,一OC CH-0 CH,一O一C triolein.mp-4 C Copyright2010 Pearson Prentice Hall,Inc Unsaturated triglycerides have lower melting points because their unsaturated fatty acids do not pack as well in a solid lattice. Chapter 25 9
Chapter 25 9 Fats and Oils ▪ Unsaturated triglycerides have lower melting points because their unsaturated fatty acids do not pack as well in a solid lattice
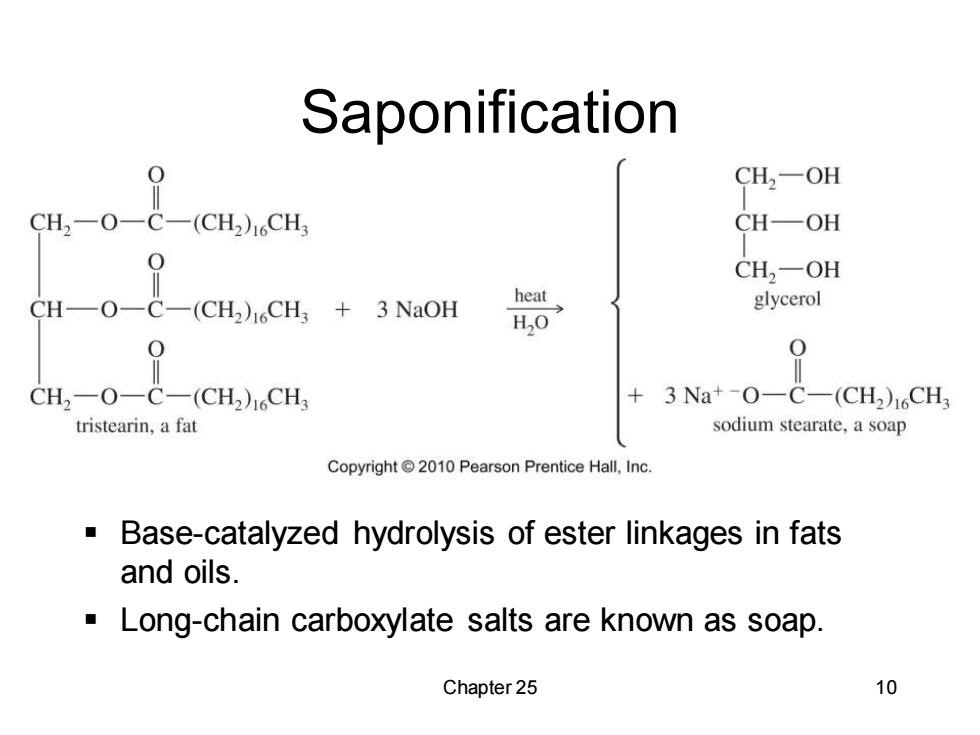
Saponification H-0二69 CH2一OH (CH2)1CH CH—OH CH2-OH CH-O-C-(CH2)16CH3 +3 NaOH heat glycerol HO 0 0 CH2-O-C-(CH2)1CHs 3Na+-O一C-(CH2)16CH3 tristearin,a fat sodium stearate,a soap Copyright2010 Pearson Prentice Hall,Inc. Base-catalyzed hydrolysis of ester linkages in fats and oils. Long-chain carboxylate salts are known as soap. Chapter 25 10
Chapter 25 10 Saponification ▪ Base-catalyzed hydrolysis of ester linkages in fats and oils. ▪ Long-chain carboxylate salts are known as soap