
Mechanism of Desulfonation S03 H+ S03 (resonance-delocalized) (SO3+H2O H2SO) Copyright 2010 Pearson Prentice Hall,Inc. In the desulfonation reaction,a proton adds to the ring (the electrophile)and loss of sulfur trioxide gives back benzene. Chapter 17 16
Chapter 17 16 Mechanism of Desulfonation ▪ In the desulfonation reaction, a proton adds to the ring (the electrophile) and loss of sulfur trioxide gives back benzene
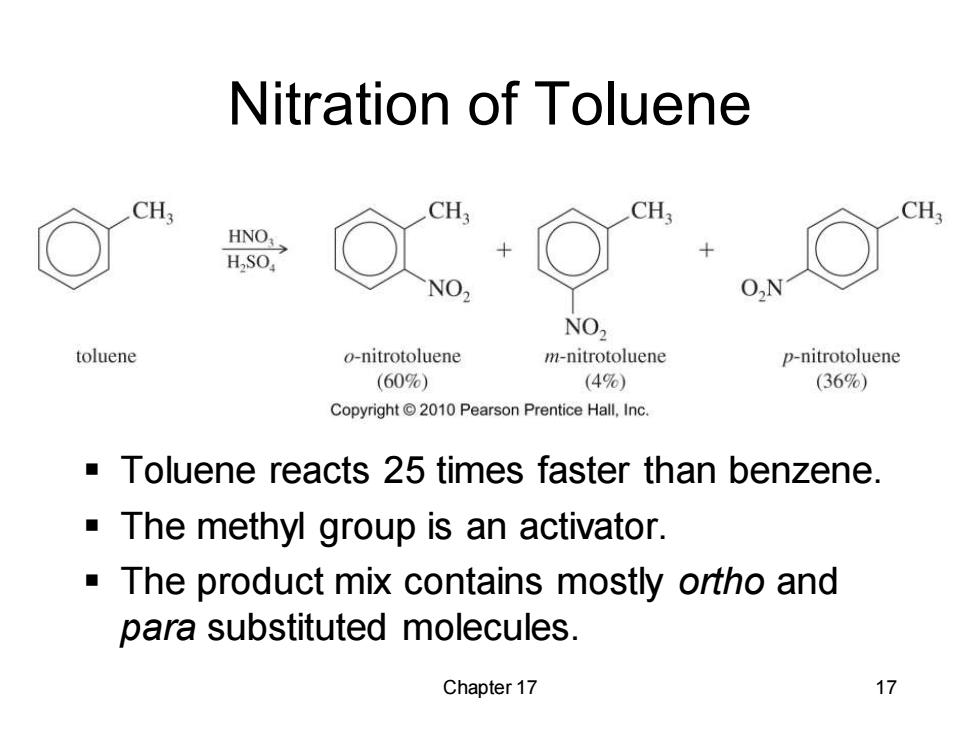
Nitration of Toluene CH; CH: HNO: HSO, NO O,N NO2 toluene o-nitrotoluene m-nitrotoluene p-nitrotoluene (60%) (4%) (36%) Copyright 2010 Pearson Prentice Hall,Inc. -Toluene reacts 25 times faster than benzene. The methyl group is an activator. -The product mix contains mostly ortho and para substituted molecules. Chapter 17 17
Chapter 17 17 Nitration of Toluene ▪ Toluene reacts 25 times faster than benzene. ▪ The methyl group is an activator. ▪ The product mix contains mostly ortho and para substituted molecules
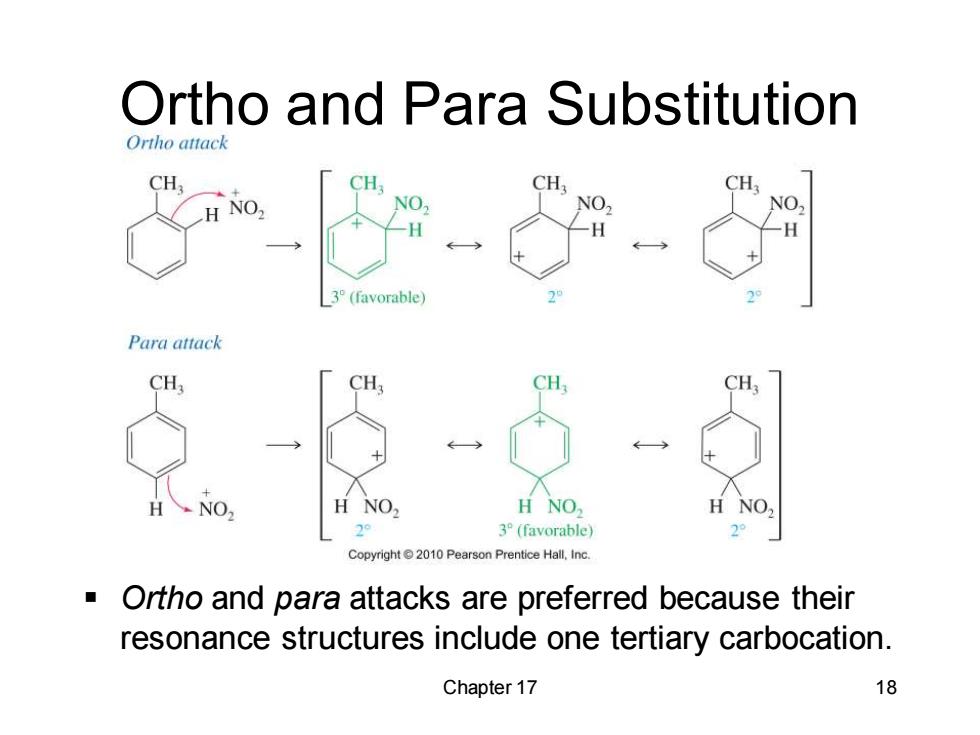
Ortho and Para Substitution Ortho attack CH CH CH, H NO, NO 3°(favorable) 2 Para attack CH3 CH: CH CH H NO, NO 2° 3°(favorable) 2 Copyright010 Pearson Prentice Hall,Inc. Ortho and para attacks are preferred because their resonance structures include one tertiary carbocation. Chapter 17 18
Chapter 17 18 Ortho and Para Substitution ▪ Ortho and para attacks are preferred because their resonance structures include one tertiary carbocation

Energy Diagram H benzene meta CH ortho,para H CH: NO2 CH; H K3aua NO2 H NO2 ++NO2 reaction coordinate Copyright2010 Pearson Prentice Hall,Inc. Chapter 17 19
Chapter 17 19 Energy Diagram

Meta Substitution Meta attack CH; CH CH NO, -NO NO H H H H NO2 2° 2° Copyright 2010 Pearson Prentice Hall,Inc. When substitution occurs at the meta position,the positive charge is not delocalized onto the tertiary carbon,and the methyl groups has a smaller effect on the stability of the sigma complex. Chapter 17 20
Chapter 17 20 Meta Substitution ▪ When substitution occurs at the meta position, the positive charge is not delocalized onto the tertiary carbon, and the methyl groups has a smaller effect on the stability of the sigma complex