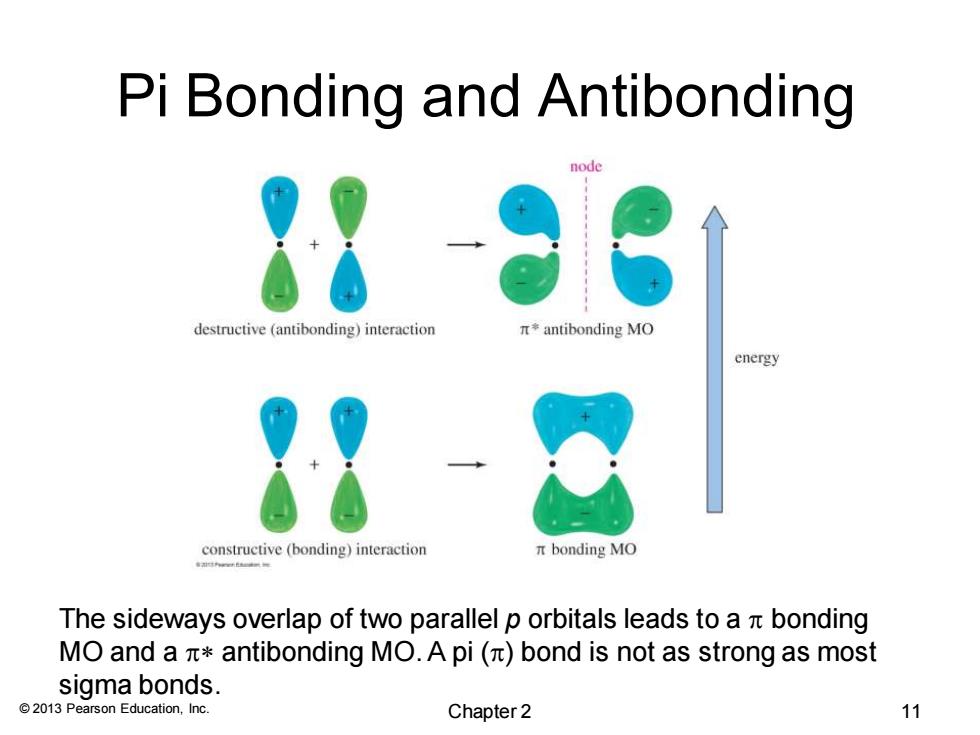
Pi Bonding and Antibonding node destructive (antibonding)interaction π*antibonding MO energy constructive(bonding)interaction bonding MO The sideways overlap of two parallel p orbitals leads to a t bonding MO and a antibonding MO.A pi (bond is not as strong as most sigma bonds. 2013 Pearson Education,Inc. Chapter 2 11
© 2013 Pearson Education, Inc. Pi Bonding and Antibonding The sideways overlap of two parallel p orbitals leads to a p bonding MO and a p* antibonding MO. A pi (p) bond is not as strong as most sigma bonds. Chapter 2 11
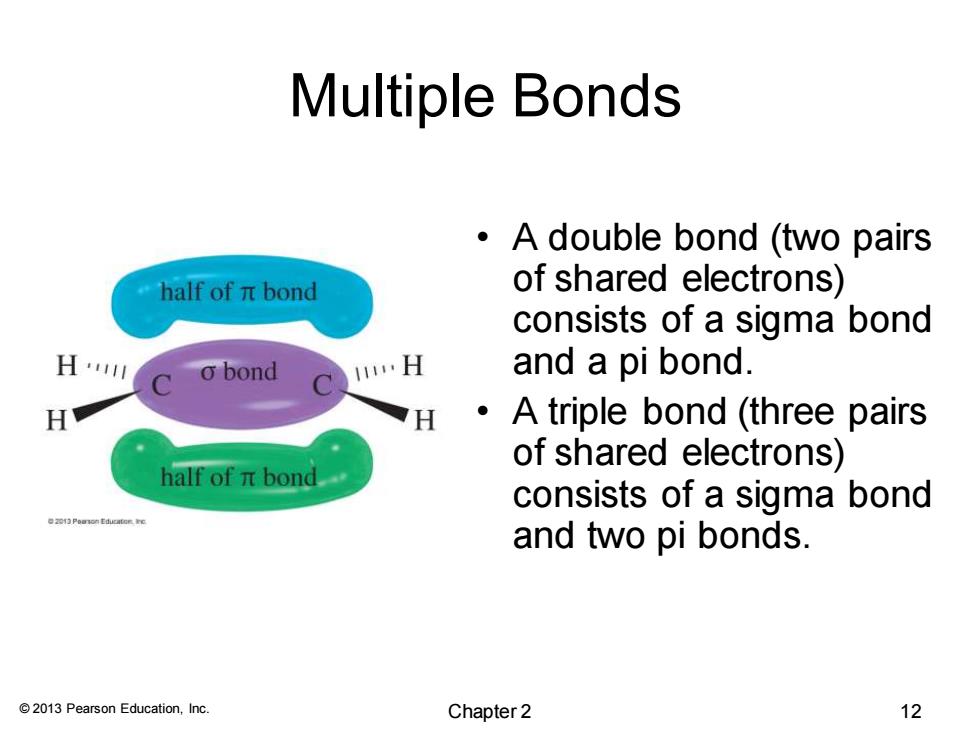
Multiple Bonds A double bond (two pairs "half ofπbond of shared electrons) consists of a sigma bond H4川 o bond 1…H and a pi bond. H H A triple bond (three pairs of shared electrons) half ofπbond consists of a sigma bond and two pi bonds. 2013 Pearson Education,Inc. Chapter2 12
© 2013 Pearson Education, Inc. Multiple Bonds • A double bond (two pairs of shared electrons) consists of a sigma bond and a pi bond. • A triple bond (three pairs of shared electrons) consists of a sigma bond and two pi bonds. Chapter 2 12
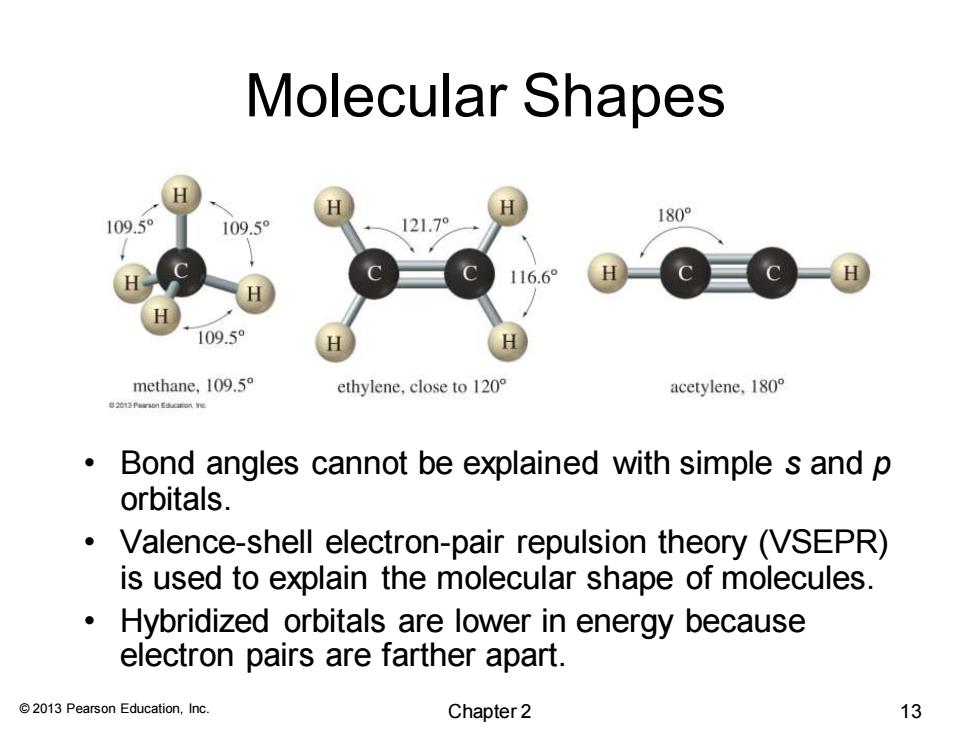
Molecular Shapes 180° 109.5° 109.5° 121.7° 116.6° 109.5 methane,109.5 ethylene,close to 120 acetylene,180 Bond angles cannot be explained with simple s and p orbitals. Valence-shell electron-pair repulsion theory (VSEPR) is used to explain the molecular shape of molecules. Hybridized orbitals are lower in energy because electron pairs are farther apart. 2013 Pearson Education,Inc. Chapter 2 13
© 2013 Pearson Education, Inc. Molecular Shapes • Bond angles cannot be explained with simple s and p orbitals. • Valence-shell electron-pair repulsion theory (VSEPR) is used to explain the molecular shape of molecules. • Hybridized orbitals are lower in energy because electron pairs are farther apart. Chapter 2 13
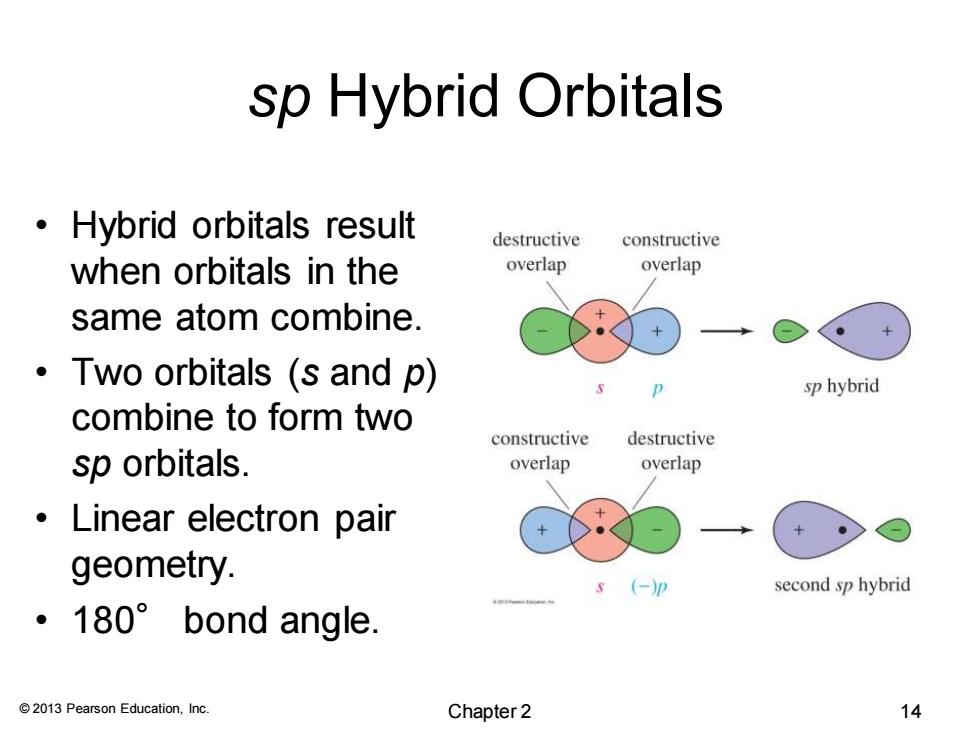
sp Hybrid Orbitals Hybrid orbitals result destructive constructive when orbitals in the overlap overlap same atom combine. ·Two orbitals(s and p) sp hybrid combine to form two constructive destructive sp orbitals. overlap overlap 。Linear electron pair geometry. s (-)p second sp hybrid ·180°bond angle. 2013 Pearson Education,Inc. Chapter 2 14
© 2013 Pearson Education, Inc. sp Hybrid Orbitals • Hybrid orbitals result when orbitals in the same atom combine. • Two orbitals (s and p) combine to form two sp orbitals. • Linear electron pair geometry. • 180° bond angle. Chapter 2 14

HINT The number of hybrid orbitals formed is always the same as the total number of s and p orbitals hybridized. 2013 Pearson Education,Inc. Chapter 2 15
© 2013 Pearson Education, Inc. The number of hybrid orbitals formed is always the same as the total number of s and p orbitals hybridized. Chapter 2 15