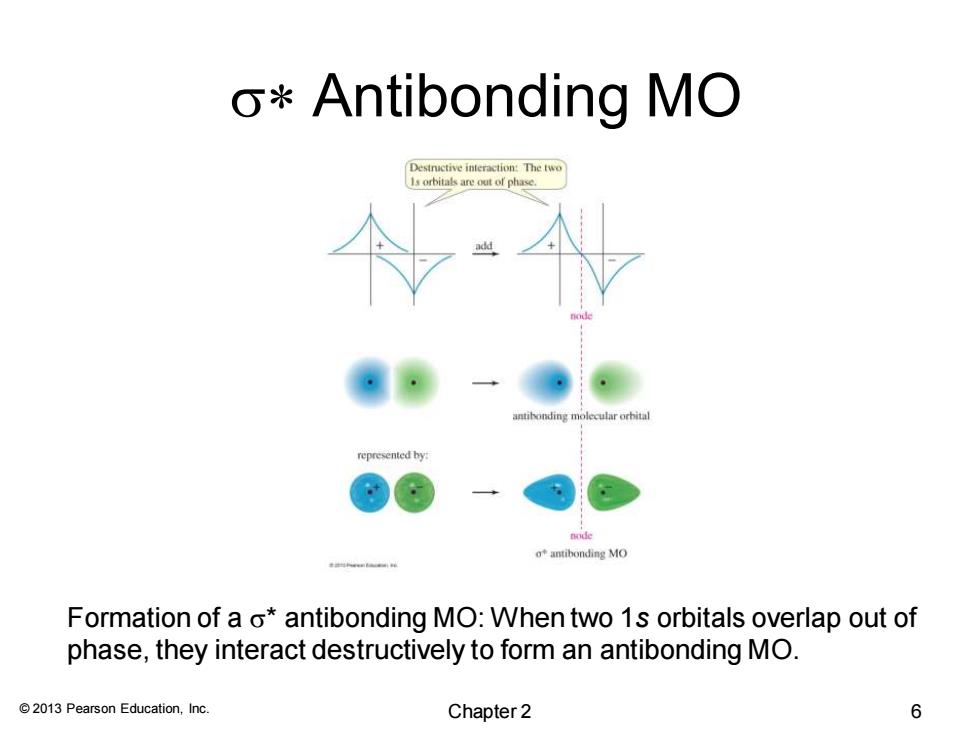
o*Antibonding MO Destructive interaction:The two 1s orbitals are out of phase. antibonding molecular orbital represented by: ot antibonding MO Formation of a o*antibonding MO:When two 1s orbitals overlap out of phase,they interact destructively to form an antibonding MO. 2013 Pearson Education,Inc. Chapter 2 6
© 2013 Pearson Education, Inc. s* Antibonding MO Formation of a s* antibonding MO: When two 1s orbitals overlap out of phase, they interact destructively to form an antibonding MO. Chapter 2 6
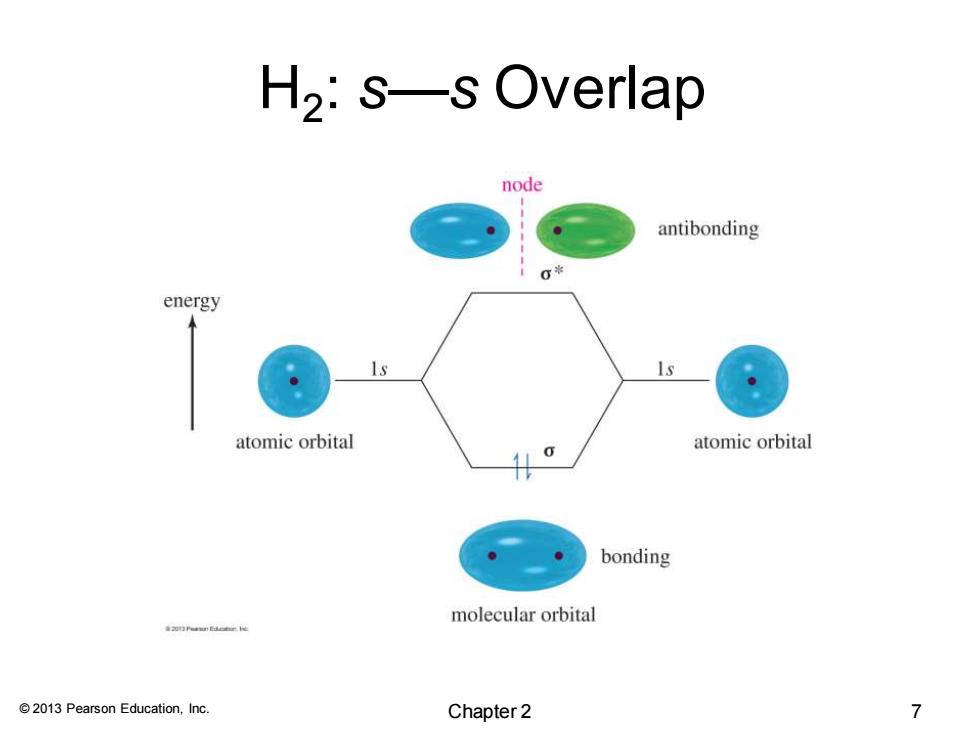
H2:s—s Overlap node antibonding 0端 energy atomic orbital atomic orbital 0 bonding molecular orbital 201 Pern Eaee 2013 Pearson Education,Inc. Chapter 2 7
© 2013 Pearson Education, Inc. H2 : s—s Overlap Chapter 2 7
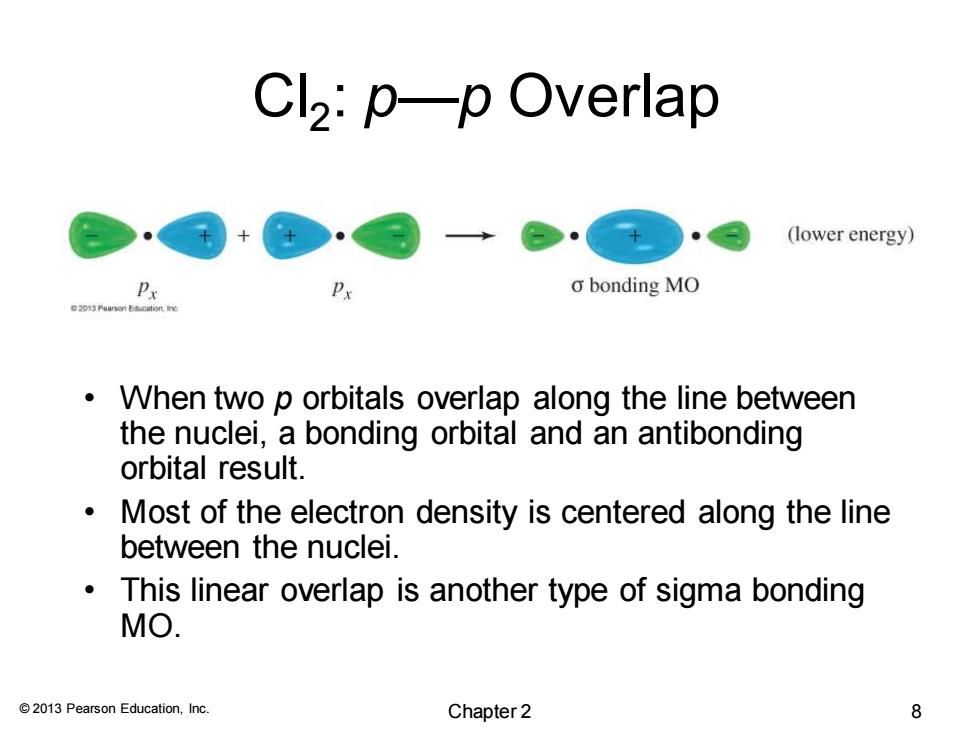
Cl2:p—p Overlap (lower energy) o bonding MO When two p orbitals overlap along the line between the nuclei,a bonding orbital and an antibonding orbital result. Most of the electron density is centered along the line between the nuclei. This linear overlap is another type of sigma bonding MO. 2013 Pearson Education,Inc. Chapter 2 8
© 2013 Pearson Education, Inc. Cl2 : p—p Overlap • When two p orbitals overlap along the line between the nuclei, a bonding orbital and an antibonding orbital result. • Most of the electron density is centered along the line between the nuclei. • This linear overlap is another type of sigma bonding MO. Chapter 2 8
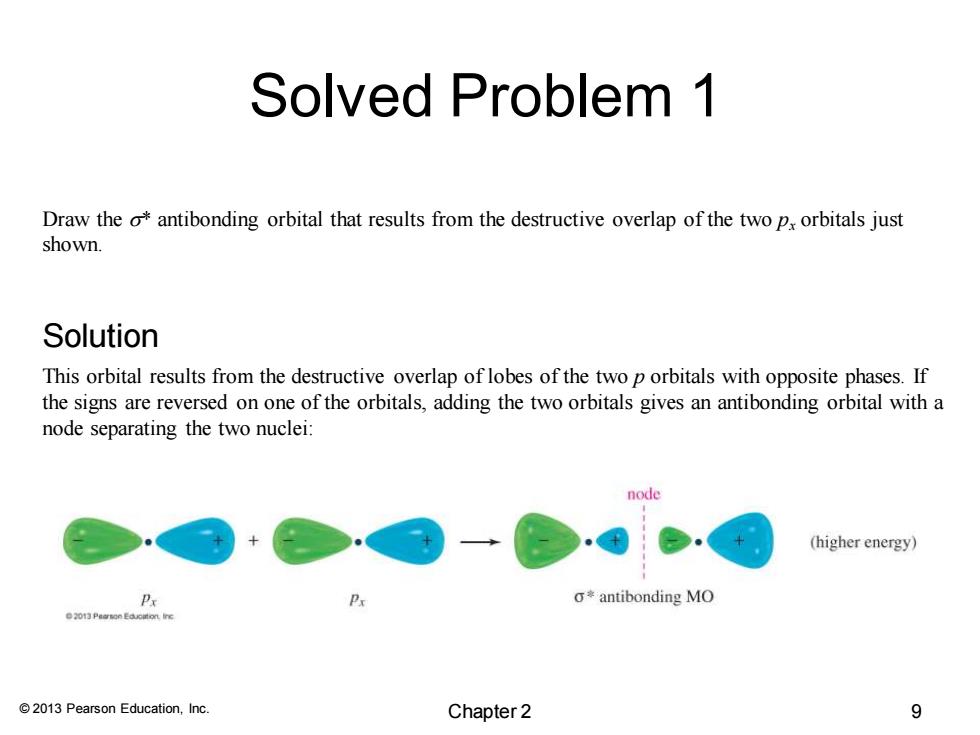
Solved Problem 1 Draw the o*antibonding orbital that results from the destructive overlap of the two px orbitals just shown. Solution This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases.If the signs are reversed on one of the orbitals,adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: node (higher energy) Px g来antibonding MO 0 Pearson Eacition ire 2013 Pearson Education,Inc. Chapter 2 9
© 2013 Pearson Education, Inc. Solved Problem 1 Draw the s* antibonding orbital that results from the destructive overlap of the two px orbitals just shown. This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases. If the signs are reversed on one of the orbitals, adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: Solution Chapter 2 9
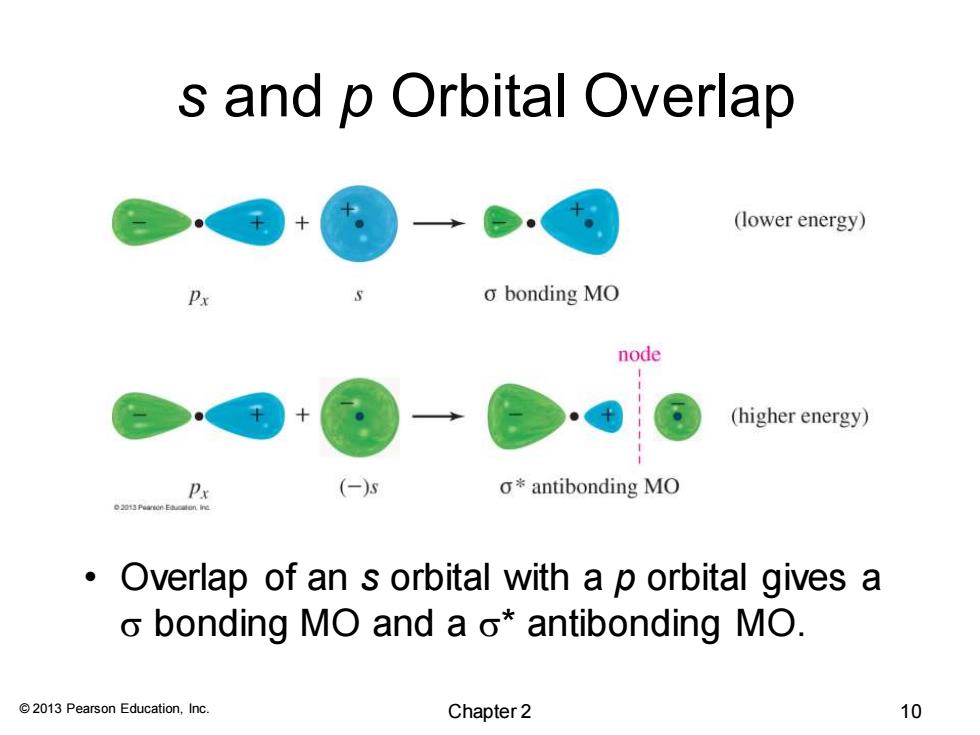
s and p Orbital Overlap (lower energy) o bonding MO node (higher energy) Px (-) o*antibonding MO 。 Overlap of an s orbital with a p orbital gives a o bonding MO and a o*antibonding MO. 2013 Pearson Education,Inc. Chapter 2 10
© 2013 Pearson Education, Inc. s and p Orbital Overlap • Overlap of an s orbital with a p orbital gives a s bonding MO and a s* antibonding MO. Chapter 2 10