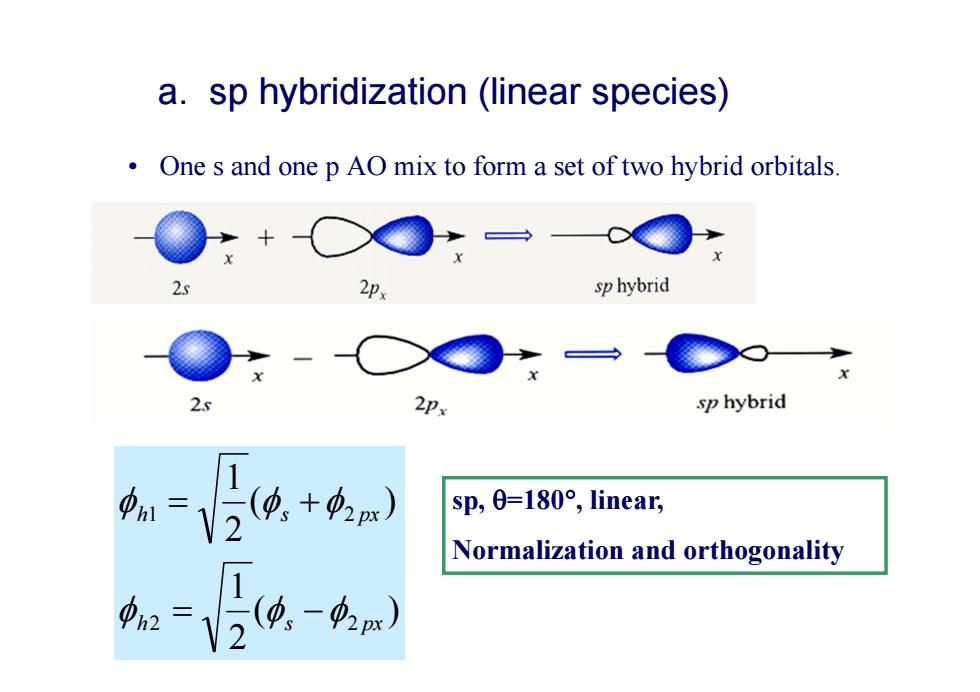
a.sp hybridization (linear species) One s and one p AO mix to form a set of two hybrid orbitals. sp hybrid 2. 2Px sp hybrid 1 sp,0=l80°,linear, Normalization and orthogonality h2
a. sp hybridization (linear species) • One s and one p AO mix to form a set of two hybrid orbitals. ( ) 2 1 ( ) 2 1 2 2 1 2 h s px h s px sp, =180 , linear, Normalization and orthogonality
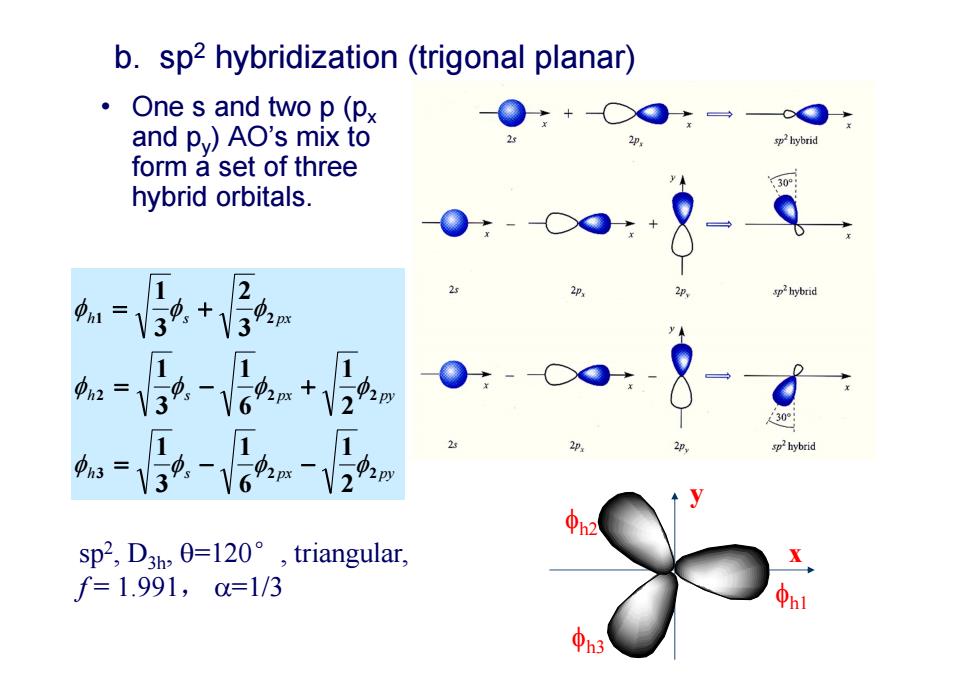
b.sp2 hybridization(trigonal planar) ·One s and two p(px and p)AO's mix to hybrid form a set of three 30 hybrid orbitals. 2 1=32+3nm =39 sp2,D3h,0=l20°,triangular, f=1.991,0=1/3
h s px py h s px py h s px 3 2 2 2 2 2 1 2 2 1 6 1 3 1 2 1 6 1 3 1 3 2 3 1 • One s and two p (px and py) AO’s mix to form a set of three hybrid orbitals. b. sp2 hybridization (trigonal planar) sp2, D3h, =120°, triangular, f = 1.991, =1/3 h1 h2 h3 x y
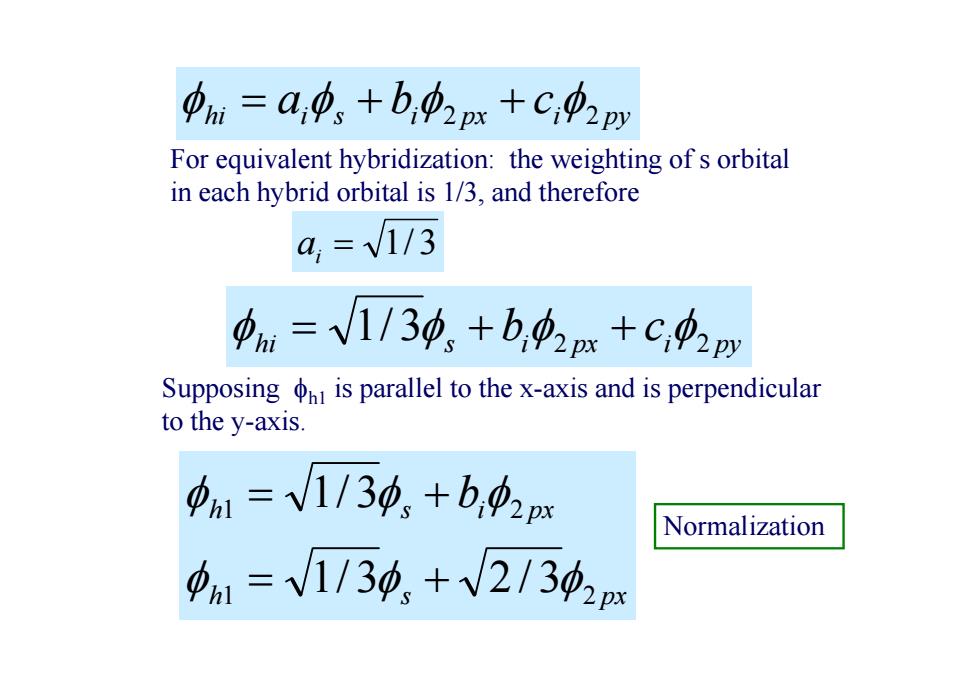
中i=a,中、+b,42m+C,02py For equivalent hybridization:the weighting of s orbital in each hybrid orbital is 1/3,and therefore a,=V1/3 中ni=V1/30,+b,中2x+C4m Supposing is parallel to the x-axis and is perpendicular to the y-axis 中1=V1/30,+b,2px Normalization a1=V1/30,+V21302x
hi i s i px i py a b c 2 2 For equivalent hybridization: the weighting of s orbital in each hybrid orbital is 1/3, and therefore 1/ 3 i a Supposing h1 is parallel to the x-axis and is perpendicular to the y-axis. h s px h s i px b 1 2 1 2 1/ 3 2 / 3 1/ 3 Normalization hi s i px i py b c 3 2 2 1/
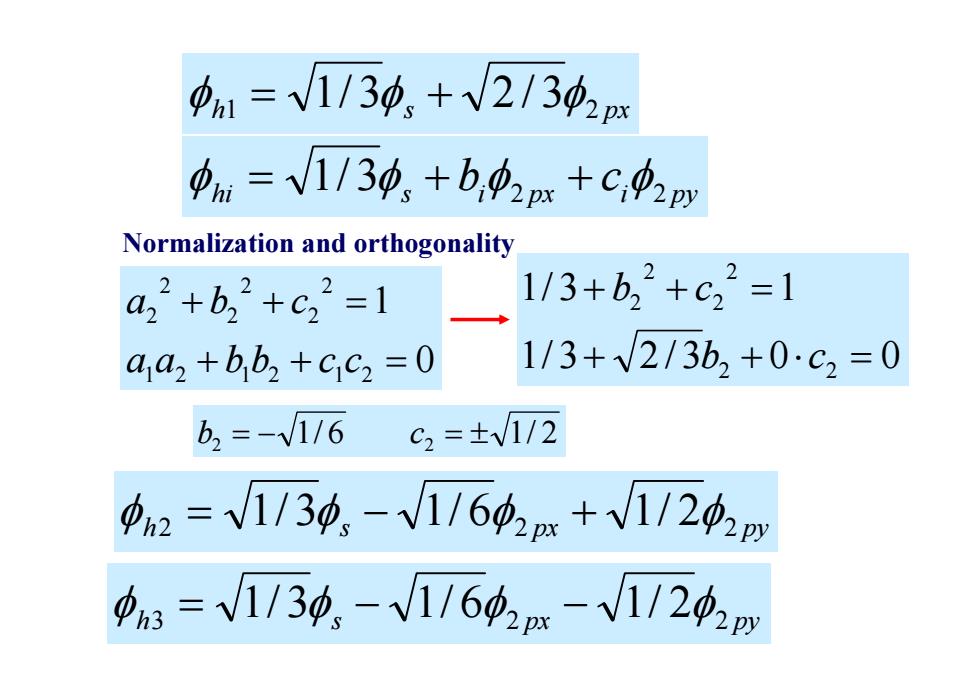
n1=V1/30,+V2/342p 中ni=V1/34,+b,02m+C,02py Normalization and orthogonality a,2+b,2+c32=1 1/3+b,2+c,2=1 aaz +bb2 +cc2 =0 1/3+V2/3b2+0c=0 b2=-116 c2=±V1/2 a2=V1/3功,-V1/642m+V1/20y =V1/30,-V1/60m-V1/24,m
hi s i px i py b c 3 2 2 1/ h1 s 2 px 1/ 3 2 / 3 Normalization and orthogonality 1/ 3 2 / 3 0 0 1/ 3 1 2 2 2 2 2 2 b c b c 0 1 1 2 1 2 1 2 2 2 2 2 2 2 a a b b c c a b c b2 1/ 6 c2 1/ 2 h2 s 2 px 2 py 1/ 3 1/ 6 1/ 2 h3 s 2 px 2 py 1/ 3 1/ 6 1/ 2

c.sp3 hybrides (tetrahedral) H equivalent H:C:H =s+px+p+p:1 hybridization H along (x,y,z) o=1/4 2 =2{s-Px-P,+P] along(-x,-y,z) sB+P,-P. along (-x,y,-Z) h4 ,s+p,-D,-p One s and 3 p AO's mix to form a set of along (x,-y.-Z) four hybrid sp3 orbitals. CH,SiH,GeH:PX,SO-,PO
H C H H H along (x, y,z) 1 [ ] 2 1 h p x p y p z s along (-x,-y,z) 2 [ ] 2 1 h p x p y p z s along (-x, y,-z) 3 [ ] 2 1 h p x p y p z s along (x,-y,-z) [ ] 2 1 h 4 p x p y p z s h1 h2 h4 h3 z • One s and 3 p AO’s mix to form a set of four hybrid sp 3 orbitals. equivalent hybridization =1/4 c. sp 3 hybrides (tetrahedral) 3 4 2 4 4 4 4 4 CH , SiH ,GeH , PX , SO , PO x y