Chapter 6 Polyatomic molecules (II) 6.1 Electron-deficient multi-center bonds and electron-deficient molecules 6.1.1 Boranes and their relatives
Chapter 6 Polyatomic molecules (II) 6.1 Electron-deficient multi-center bonds and electron-deficient molecules 6.1.1 Boranes and their relatives
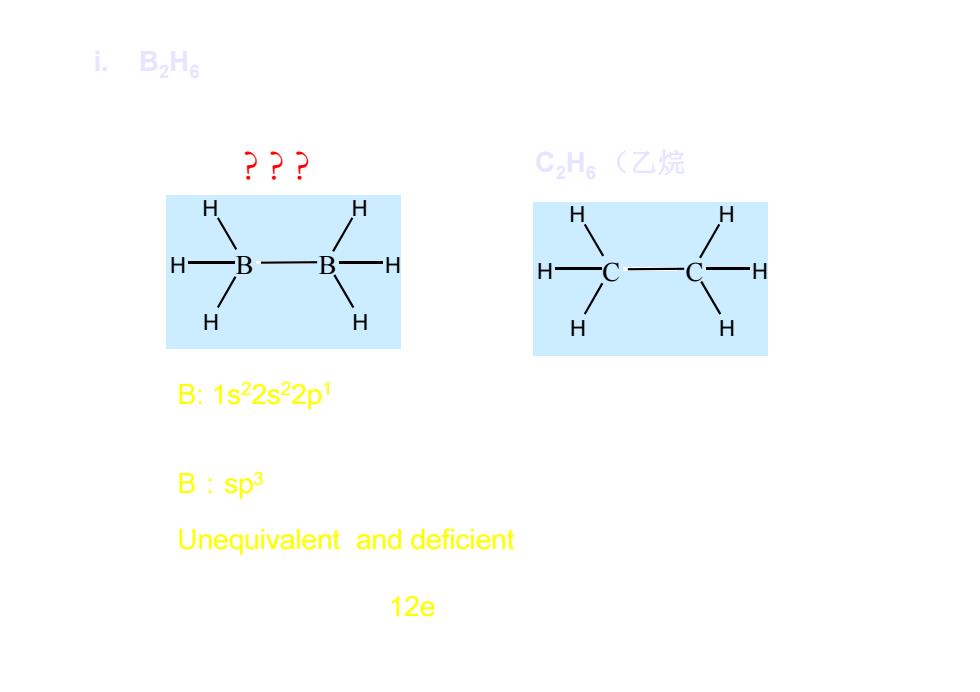
i.B2He ??? CH。(乙烷 B:1s22s22p B:sp3 Unequivalent and deficient 12e
? ? ? B H H H B H H H i. B2H6 C H H H C H H H C2H6 (乙烷 ethane) (乙硼烷 diborane; boroethane ) B: 1s22s22p1 C: 1s22s22p2 Hybridization orbitals B:sp3 C: sp3 Unequivalent and deficient equivalent orbitals valence electron:12e 14e
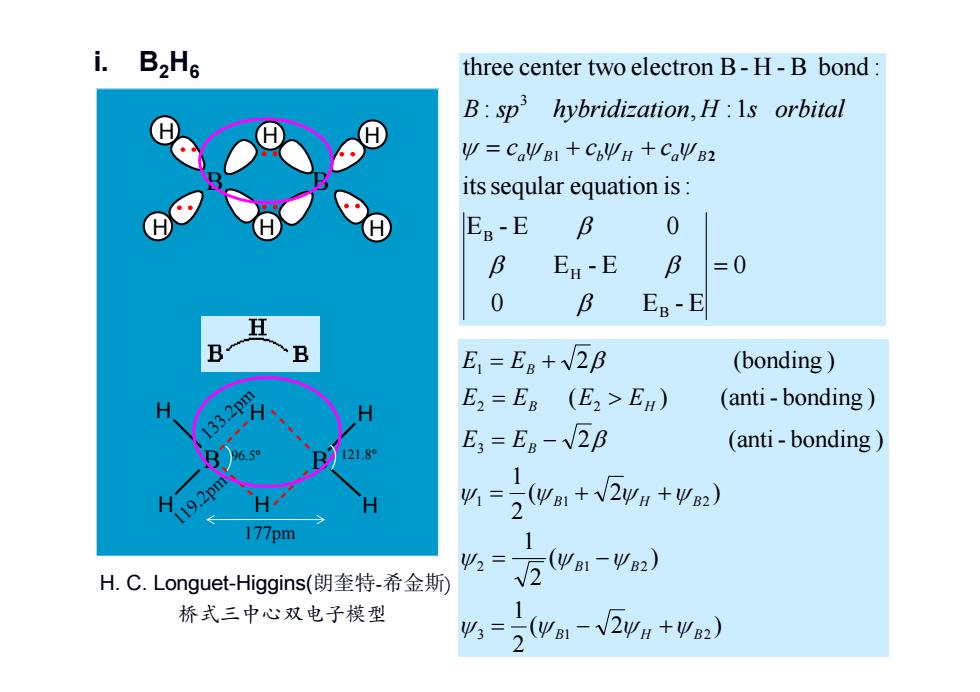
i.B2He three center two electron B-H-B bond: B:sp hybridization,H:Is orbital y=CaΨB1+CbΨH+CaΨB2 its seqular equation is: H E8-E B 0 B En-E B =0 0 B E8-E B B E=Eg+2B (bonding) E2=Eg (E2>En) (anti-bonding) 33 E;=E8-2B (anti-bonding) 12189 1 19.2pm 必1= (ya1+V2yH+y2) 2 177pm 1 Ψ2= 5(ΨB1-ΨB2) H.C.Longuet-Higgins(朗奎特-希金斯 桥式三中心双电子模型 1 =2 (9B1-V2yH+Ψ2)
p125 HMO method 0 0 E - E E - E E - E 0 itsseqular equation is: : , :1 three center two electron B- H - B bond : B H B 1 3 a B b H a B2 c c c B sp hybridization H s orbital i. B2H6 ( 2 ) 21 ( ) 21 ( 2 ) 21 2 (anti - bonding ) ( ) (anti - bonding ) 2 (bonding ) 3 1 2 2 1 2 1 1 2 3 2 2 1 B H B B B B H B B B H B E E E E E E E E B H H H H B H H 96.5° 121.8° B H H H H H H B H. C. Longuet-Higgins(朗奎特-希金斯) 177pm 桥式三中心双电子模型
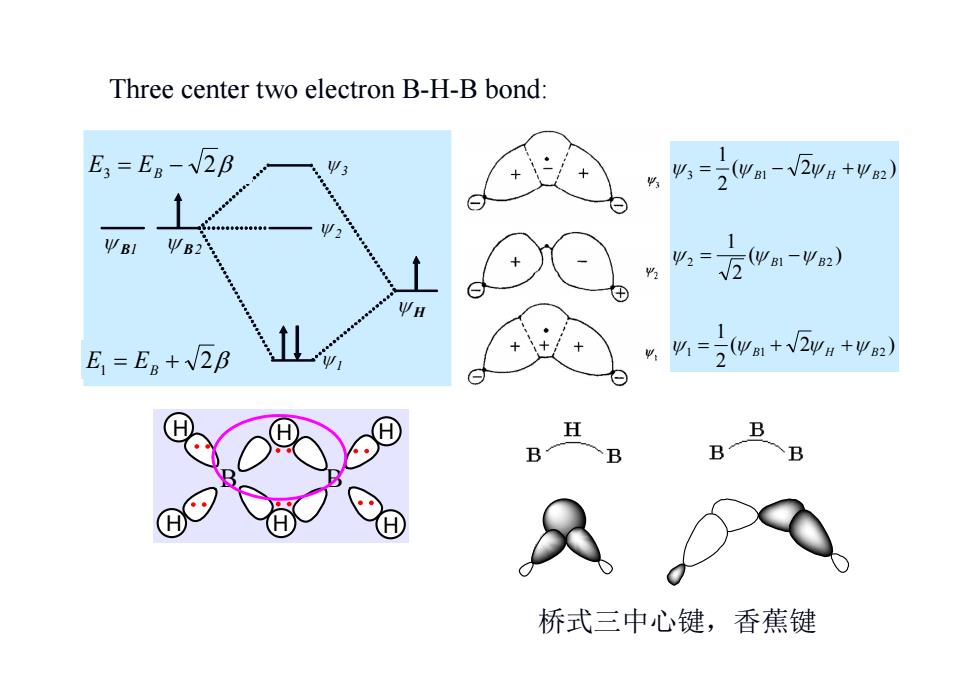
Three center two electron B-H-B bond: E3 E8-2B 1 Ψ3 %=2wa-V2y4+42) Ψ ΨB1 ΨB2 1 业2= (Ψ1-Ψ82) √2 E=Eg+2B %=2wam+V2y+2) H B B 桥式三中心键,香蕉键
B H H H H H H B Three center two electron B-H-B bond: ( 2 ) 21 ( ) 21 ( 2 ) 21 1 1 2 2 1 2 3 1 2 B H B B B B H B B1 B2 H 1 2 3 E1 EB 2 E3 EB 2 桥式三中心键,香蕉键
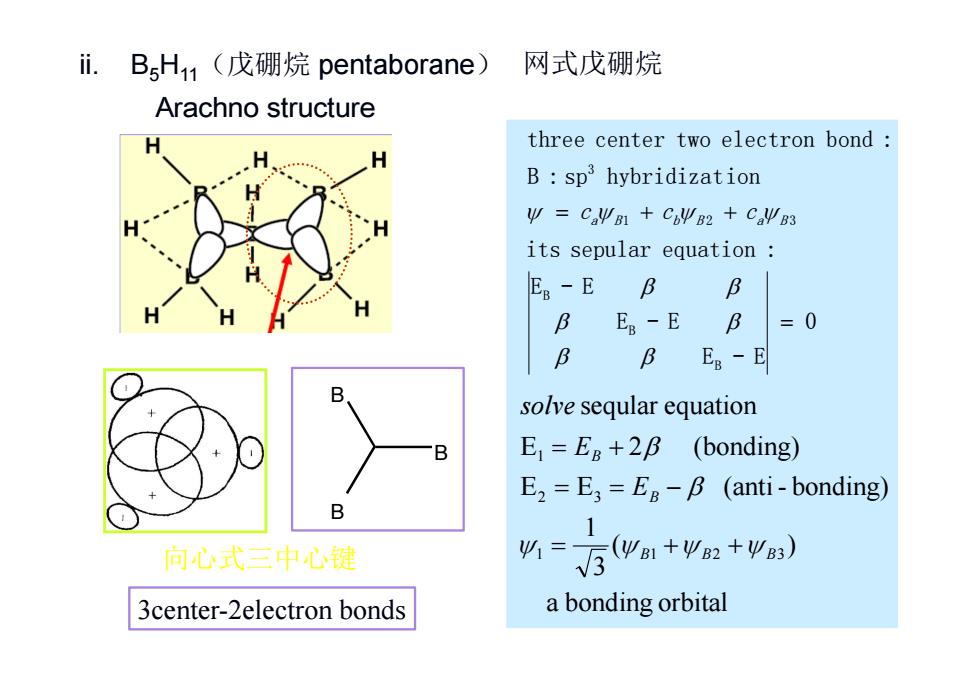
i.BsH1(戊硼烷pentaborane) 网式戊硼烷 Arachno structure three center two electron bond B:sp3hybridization Ψ=CaΨ1+CΨB2+CΨB3 its sepular equation ER-E 6 B B Es-E B =0 B B Ep-E solve seqular equation B E=Eg+28 (bonding) E2 =E3=E8-B (anti-bonding) B 1 向心式中心键 %=后wm+w:+wa) 3center-2electron bonds a bonding orbital
ii. B5H11(戊硼烷 pentaborane) 0 E - E E - E E - E its sepular equation : B : sp hybridization three center two electron bond : B B B 1 2 3 3 ca B cb B ca B a bonding orbital ( ) 31 E E (anti - bonding) E 2 (bonding) seqular equation 1 1 2 3 2 3 1 B B B B B E E solve B B B 3center-2electron bonds 向心式三中心键 Arachno structure 网式戊硼烷