
What's a chemical bond? Chemical Bonding
What’s a chemical bond? Chemical Bonding
Quantum mechanical theory for description of molecular structures and chemical bondings Molecular Orbital (MO)Theory a)Proposed by Hund,Mulliken,Lennard-Jones et al.in 1930s. b)Further developments by Slater,Huckel and Pople et al c)MO-based softwares are widely used nowaday,e.g.,Gaussian Valence Bond (VB)Theory a)Proposed by Heitler and London 1930s,further developments by Pauling and Slater et al. b)Programmed in later 1980s,e.g.,latest development--XMVB! Density Functional Theory a)Proposed by Kohn et al. b)DFT-implemented QM softwares are widely used,e.g.,Gaussian
Quantum mechanical theory for description of molecular structures and chemical bondings • Molecular Orbital (MO) Theory a) Proposed by Hund, Mulliken, Lennard-Jones et al. in 1930s. b) Further developments by Slater, Hückel and Pople et al. c) MO-based softwares are widely used nowaday, e.g., Gaussian • Valence Bond (VB) Theory a) Proposed by Heitler and London 1930s, further developments by Pauling and Slater et al. b) Programmed in later 1980s, e.g., latest development--XMVB! • Density Functional Theory a) Proposed by Kohn et al. b) DFT-implemented QM softwares are widely used, e.g., Gaussian

Slater Pauling Kohn 卢嘉锡
Slater Pauling Kohn 卢嘉锡
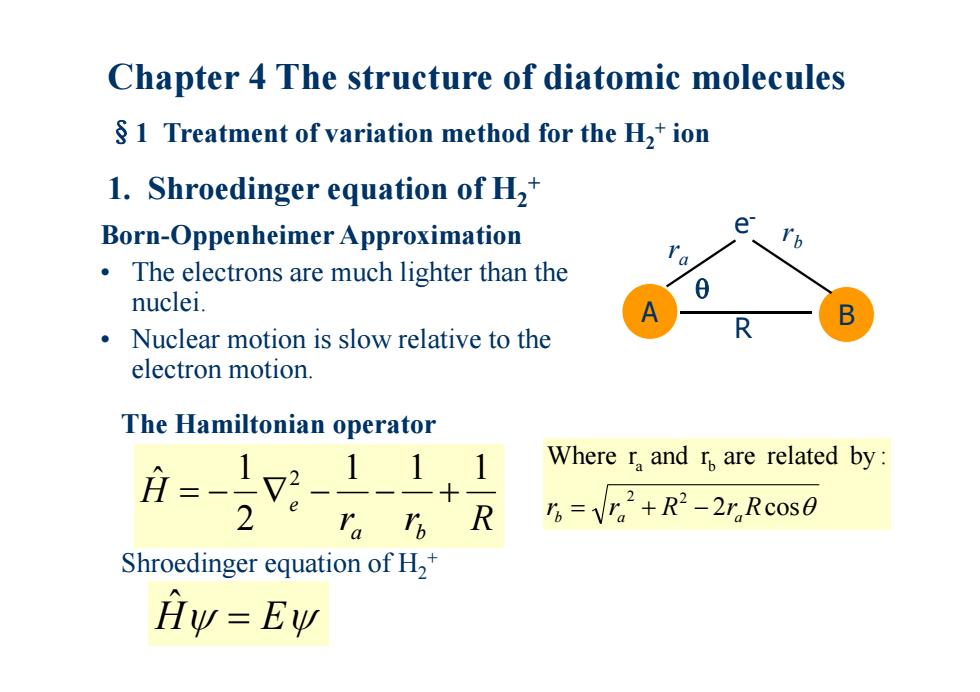
Chapter 4 The structure of diatomic molecules $1 Treatment of variation method for the H,+ion 1.Shroedinger equation of H,* Born-Oppenheimer Approximation e The electrons are much lighter than the 0 nuclei. Nuclear motion is slow relative to the R electron motion. The Hamiltonian operator 月=-12-111 Where r and r are related by: 2 ra I R 5=V.2+R2-2r.Rc0s0 Shroedinger equation of H2 Hy=Ew
Chapter 4 The structure of diatomic molecules §1 Treatment of variation method for the H 2 + ion 1. Shroedinger equation of H 2 + Born-Oppenheimer Approximation • The electrons are much lighter than the nuclei. • Nuclear motion is slow relative to the electron motion. r r R H a b e 1 1 1 2 1 ˆ 2 The Hamiltonian operator 2 cos Where r and r are related by : 2 2 a b rb ra R ra R A B e- r b r a R Hˆ E Shroedinger equation of H 2 +
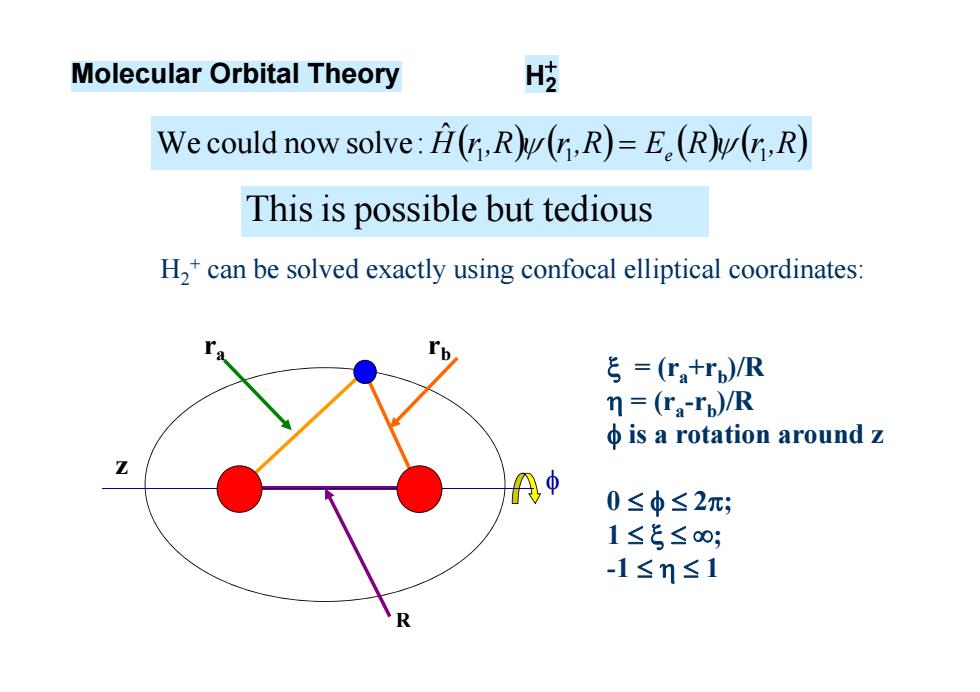
Molecular Orbital Theory H歧 We could now solve:H(,R(,R)=E.(R(r,R) This is possible but tedious H,+can be solved exactly using confocal elliptical coordinates: ξ=(Ta+rp)/R =(ra-rp)/R φis a rotation around z A中 0≤φ≤2; 1≤5≤0; -1≤m≤1
H2+ can be solved exactly using confocal elliptical coordinates: ra rb z = (ra+rb)/R = (ra-rb)/R is a rotation around z 0 2; 1 ; -1 1 R Molecular Orbital Theory H2 Hr,R r,R E R r,R 1 1 e 1 ˆ We could now solve: Thisis possible but tedious