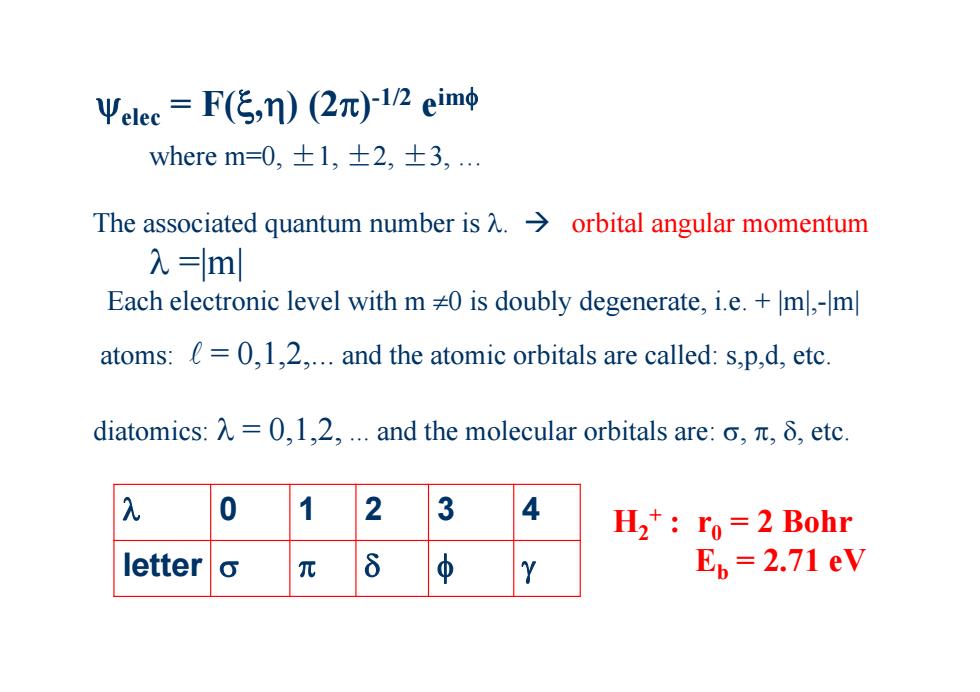
Ψelee=F(传,n)(2π)l/neim吨 where m=0,±1,±2,±3, The associated quantum number is A.>orbital angular momentum 入=ml Each electronic level with m 0 is doubly degenerate,i.e.+ml,-m atoms:=0,1,2....and the atomic orbitals are called:s.p.d,etc. diatomics:入=0,l,2,.and the molecular orbitals are:o,元,δ,etc H2*:ro=2 Bohr letter Ep 2.71 eV
elec = F(,) (2)-1/2 eim where m=0, ±1, ±2, ±3, … The associated quantum number is . orbital angular momentum =|m| Each electronic level with m 0 is doubly degenerate, i.e. + |m|,-|m| atoms: = 0,1,2,... and the atomic orbitals are called: s,p,d, etc. diatomics: = 0,1,2, ... and the molecular orbitals are: , , , etc. 0 12 3 4 letter H2+ : r0 = 2 Bohr Eb = 2.71 eV
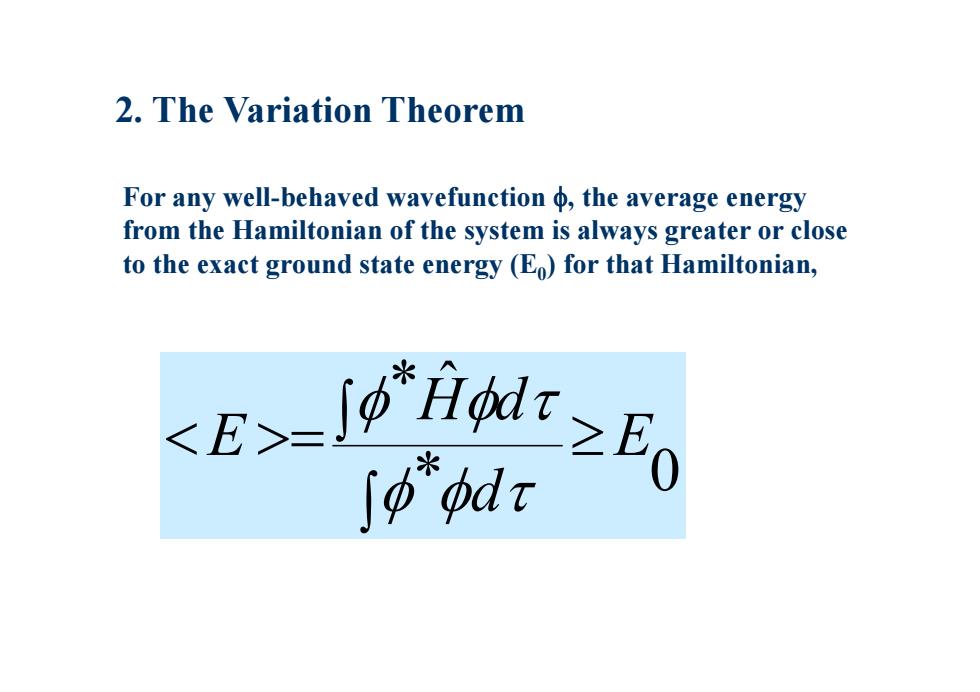
2.The Variation Theorem For any well-behaved wavefunction the average energy from the Hamiltonian of the system is always greater or close to the exact ground state energy (Eo)for that Hamiltonian, E-9≥E ∫p"pdr
2. The Variation Theorem For any well-behaved wavefunction , the average energy from the Hamiltonian of the system is always greater or close to the exact ground state energy (E 0) for that Hamiltonian, * 0 * ˆ E d H d E

Proof p(e≥Eo)its ground state(wo→Eo) p=∑cw Ψ1,Ψ2,Ψ3.consist of an A,=E,Ψ, E,≥E orthogonal normalized set of wavefunctions 「pidz <E>= 「ppdx ∫idr=∑ciw,iΣc",dr=∑c∑c∫w,iwar =∑∑ccw,E",dr=∑∑cc,E,∫w,,d fpdr=∫∑cw,∑cw,dr=∑c,∑c∫g,w,dr =∑∑cc6, ∑∑cc,Ew,"dr∑efE E=<E>= ΣΣccA ∑s ≥E
2 0 * 2 * * * * * * * * * * * * * * 0 0 0 0 E ˆ ˆ ˆ ˆ ˆ ( ) its ( ) Proof i i j i i i j ij i j i j j i j i j i j ij i j j i j j i i j j j i i i i j j i j i j i j i j j i j j i j j i i j j j i i i i i i i i i i c c E c c c c E d E c c d c c d c c d c c E d c c E d H d c H c d c c H d d H d E H E E E c E ground state E = = = 1, 2, 3 …consist of an orthogonal normalized set of wavefunctions
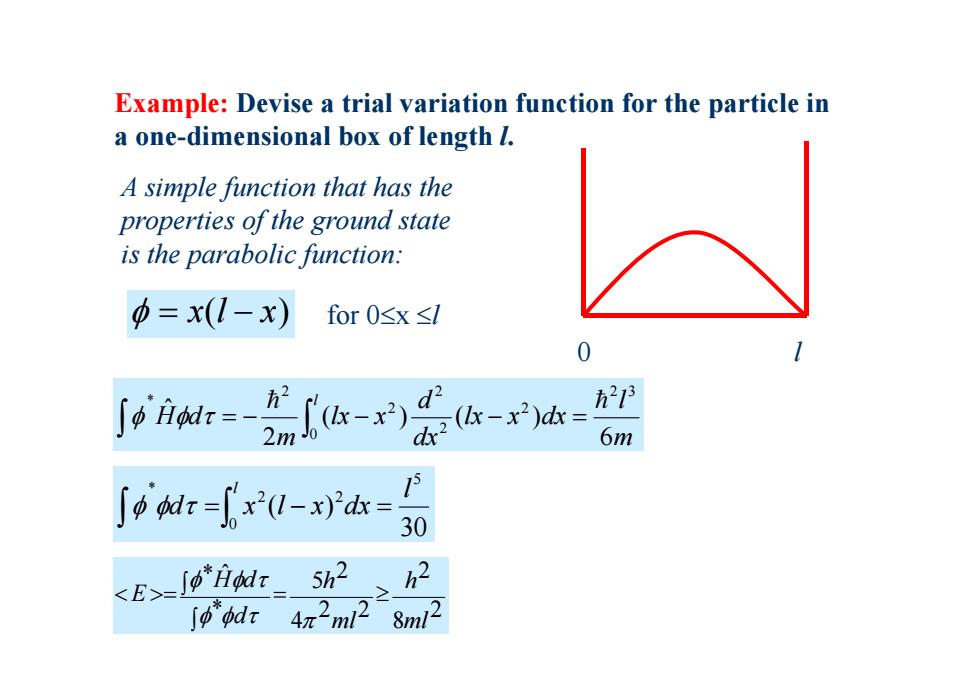
Example:Devise a trial variation function for the particle in a one-dimensional box of length I. A simple function that has the properties of the ground state is the parabolic function: 中=x(1-x) for0sx≤I 21 6m 0-a= <E>-J "Hodr 5h2 >h2 8m12
Example: Devise a trial variation function for the particle in a one-dimensional box of length l. 0 l A simple function that has the properties of the ground state is the parabolic function: x(l x) for 0x l m l lx x dx dx d lx x m H d l 6 ( ) ( ) 2 ˆ 2 3 2 2 2 0 2 2 * 30 ( ) 5 2 0 2 * l d x l x dx l 2 8 2 2 2 4 2 5 * * ˆ ml h ml h d H d E
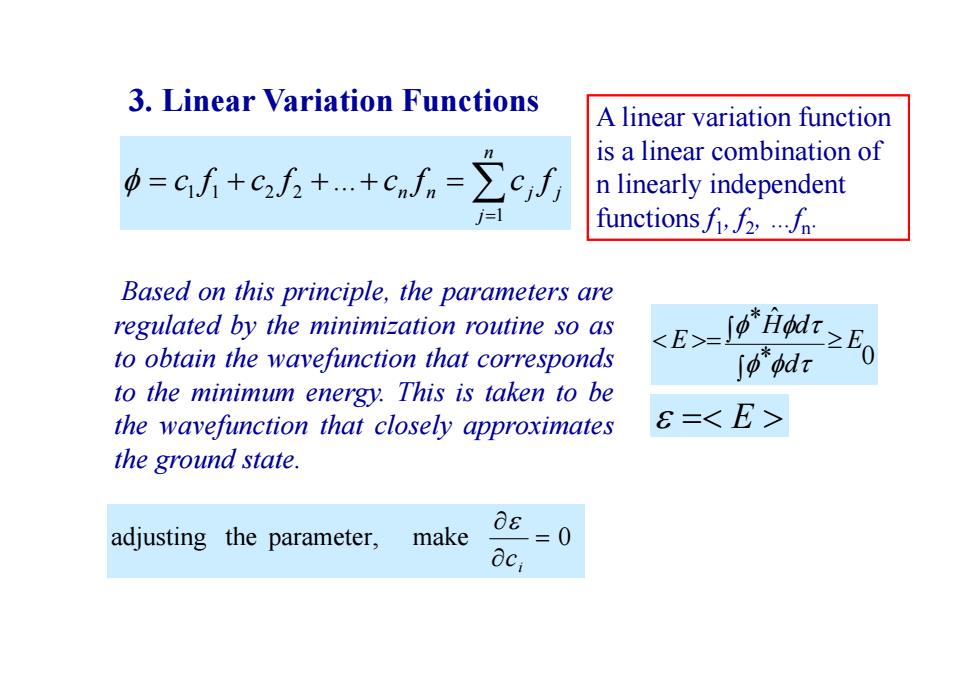
3.Linear Variation Functions A linear variation function is a linear combination of =cf+c方++cnn=∑c,f n linearly independent functionsf2... Based on this principle,the parameters are regulated by the minimization routine so as to obtain the wavefunction that corresponds 82司 J0"odr to the minimum energy.This is taken to be the wavefunction that closely approximates 6=<E> the ground state. adjusting the parameter, make 08 =0 aci
Based on this principle, the parameters are regulated by the minimization routine so as to obtain the wavefunction that corresponds to the minimum energy. This is taken to be the wavefunction that closely approximates the ground state. * 0 * ˆ E d H d E adjusting the parameter, make 0 i c E 3. Linear Variation Functions n j n n j j c f c f c f c f 1 1 1 2 2 ... A linear variation function is a linear combination of n linearly independent functions f1, f2, …fn