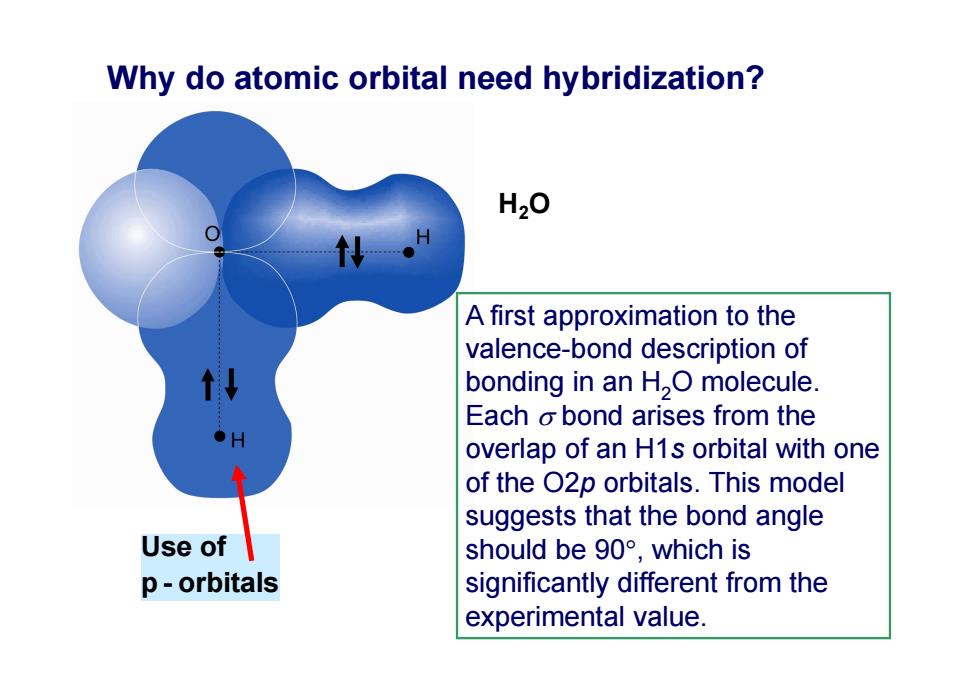
Why do atomic orbital need hybridization? H20 A first approximation to the valence-bond description of bonding in an H,O molecule. Each o bond arises from the overlap of an H1s orbital with one of the O2p orbitals.This model suggests that the bond angle Use of should be90°,which is p-orbitals significantly different from the experimental value
Use of p - orbitals H2O Why do atomic orbital need hybridization? A first approximation to the valence-bond description of bonding in an H2O molecule. Each bond arises from the overlap of an H1s orbital with one of the O2p orbitals. This model suggests that the bond angle should be 90, which is significantly different from the experimental value

104.5 b1 b2 a H20 H:03 The admixture of 2s 2p orbitals H is required. Inequivalent sp3 hybridization of O(2sp)AOs
Px Py a b 104.5 b1 b2 H O H H2O The admixture of 2s & 2p orbitals is required. Inequivalent sp3 hybridization of O(2sp) AOs
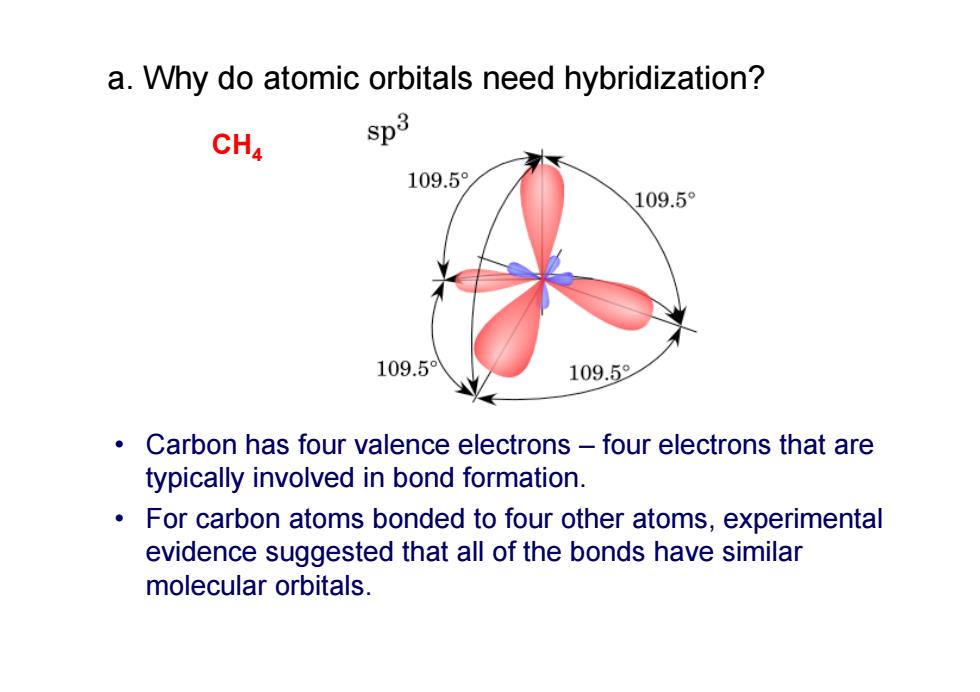
a.Why do atomic orbitals need hybridization? CH4 $p3 109.5 109.5° 109.5 109.5 Carbon has four valence electrons-four electrons that are typically involved in bond formation. For carbon atoms bonded to four other atoms,experimental evidence suggested that all of the bonds have similar molecular orbitals
• Carbon has four valence electrons – four electrons that are typically involved in bond formation. • For carbon atoms bonded to four other atoms, experimental evidence suggested that all of the bonds have similar molecular orbitals. CH 4 a. Why do atomic orbitals need hybridization?

b.How do atomic orbitals hybridize? 4。=C2,+C202m+C3py+C44p ④k=∑c4 i=i∑cx=∑ic4=∑cxi0 =∑cxE,4,=E:∑cx中 If we begin with n AO's,we must end up with n orbitals after hybridization. All n hybrids are equivalent except for directionality same energy
b. How do atomic orbitals hybridize? i i i k ik i ik i i i i ik i i ik i k ik i i k ik c E E c H H c Hc c H c ˆ ˆ ˆ ˆ h s px py pz c c c c 1 2 22 3 2 42 If we begin with n AO’s, we must end up with n orbitals after hybridization. All n hybrids are equivalent except for directionality same energy

2.Construction of hybrid orbitals
2. Construction of hybrid orbitals