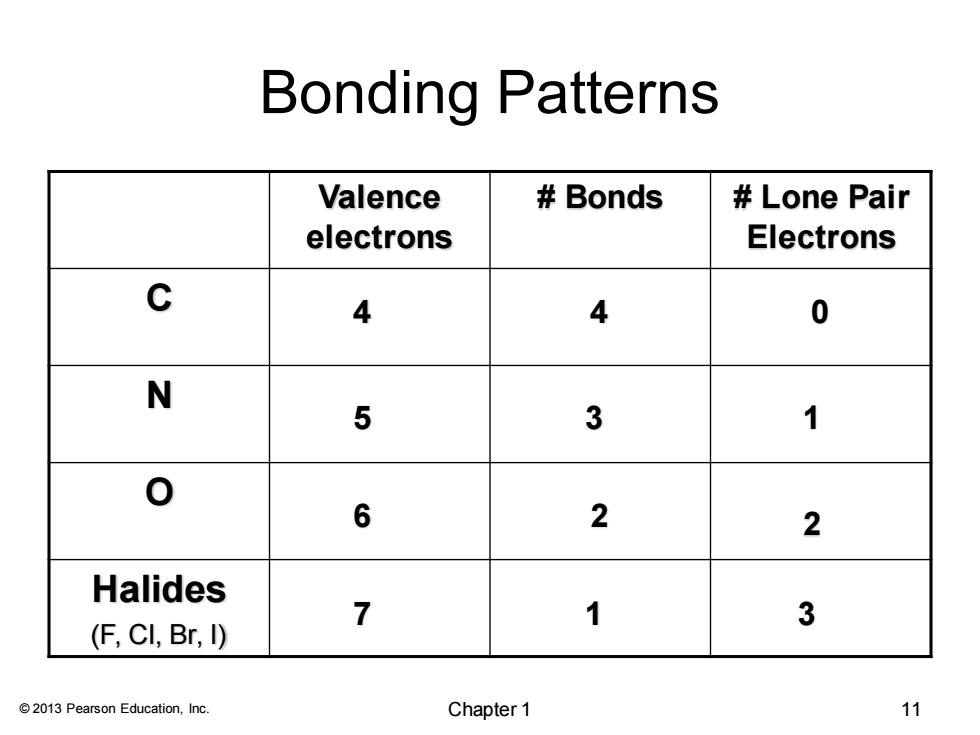
Bonding Patterns Valence Bonds Lone Pair electrons Electrons c 4 4 0 N 5 3 1 6 2 2 Halides 7 1 3 (F,CI,Br,I) 2013 Pearson Education,Inc. Chapter 1 11
© 2013 Pearson Education, Inc. Bonding Patterns Valence electrons # Bonds # Lone Pair Electrons C N O Halides (F, Cl, Br, I) 4 4 0 5 3 1 6 2 2 7 1 3 Chapter 1 11
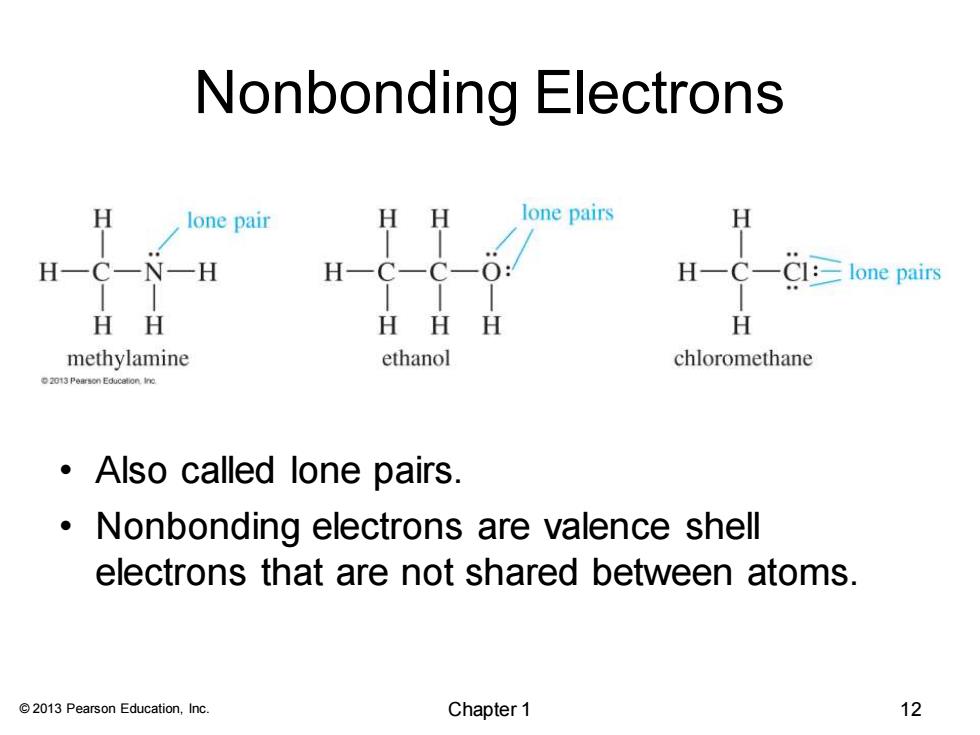
Nonbonding Electrons H lone pair HH lone pairs H H-C一N一H H-C一C- 0: H-C-Cl:=lone pairs HH HHH H methylamine ethanol chloromethane 2013 Pearson Education.ino Also called lone pairs. Nonbonding electrons are valence shell electrons that are not shared between atoms. 2013 Pearson Education,Inc. Chapter 1 12
© 2013 Pearson Education, Inc. Nonbonding Electrons • Also called lone pairs. • Nonbonding electrons are valence shell electrons that are not shared between atoms. Chapter 1 12
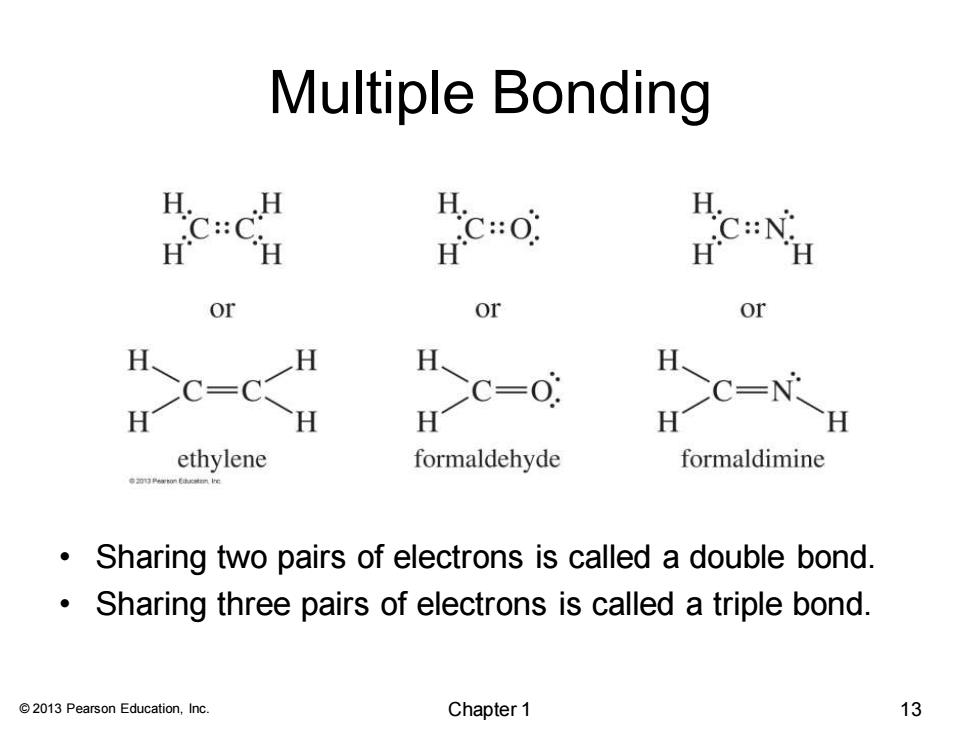
Multiple Bonding H. :: or or or H H CC=C Hc- H H 08刘 ethylene formaldehyde formaldimine Sharing two pairs of electrons is called a double bond. Sharing three pairs of electrons is called a triple bond. 2013 Pearson Education,Inc. Chapter 1 13
© 2013 Pearson Education, Inc. Multiple Bonding • Sharing two pairs of electrons is called a double bond. • Sharing three pairs of electrons is called a triple bond. Chapter 1 13

HINT Lewis structures are the way we write organic chemistry. Learning now to draw them quickly and correctly will help you throughout this course. 2013 Pearson Education,Inc. Chapter 1 14
© 2013 Pearson Education, Inc. Lewis structures are the way we write organic chemistry. Learning now to draw them quickly and correctly will help you throughout this course. Chapter 1 14

Dipole Moment 八6+ C-C1 H H chloromethane chloromethane Dipole moment is defined to be the amount of charge separation (5)multiplied by the bond length (u) 。 Charge separation is shown by an electrostatic potential map(EPM),where red indicates a partially negative region and blue indicates a partially positive region. 2013 Pearson Education,Inc. Chapter 1 15
© 2013 Pearson Education, Inc. Dipole Moment • Dipole moment is defined to be the amount of charge separation (d) multiplied by the bond length (m). • Charge separation is shown by an electrostatic potential map (EPM), where red indicates a partially negative region and blue indicates a partially positive region. Chapter 1 15