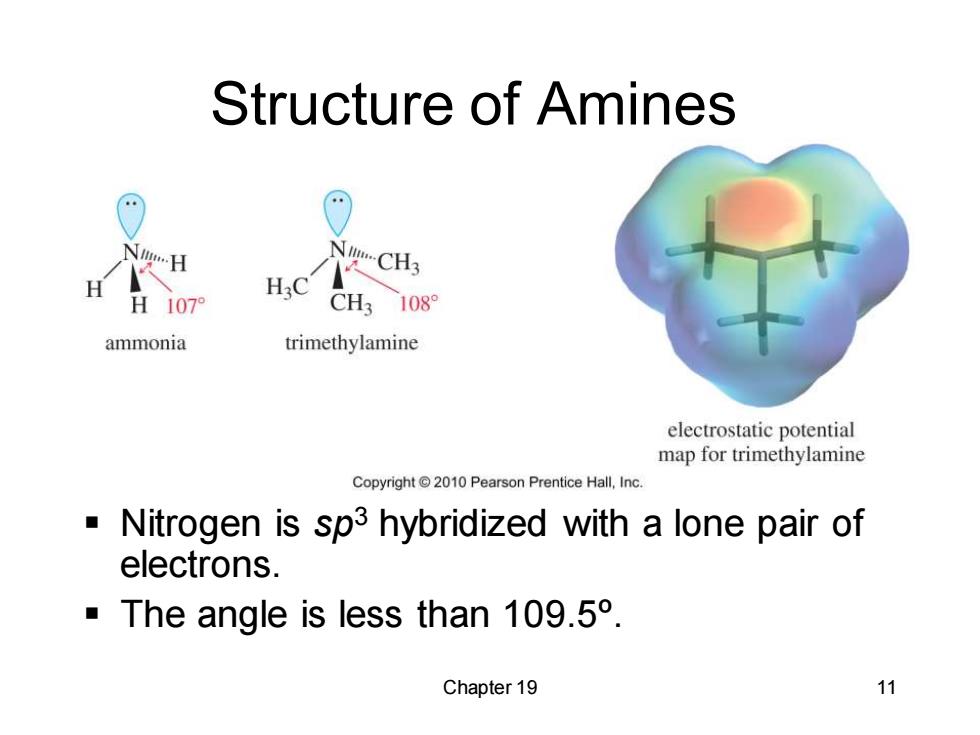
Structure of Amines H:C H 107 CH3 1089 ammonia trimethylamine electrostatic potential map for trimethylamine Copyright2010 Pearson Prentice Hall,Inc. Nitrogen is sp3 hybridized with a lone pair of electrons. The angle is less than 109.5. Chapter 19 11
Chapter 19 11 Structure of Amines ▪ Nitrogen is sp3 hybridized with a lone pair of electrons. ▪ The angle is less than 109.5º
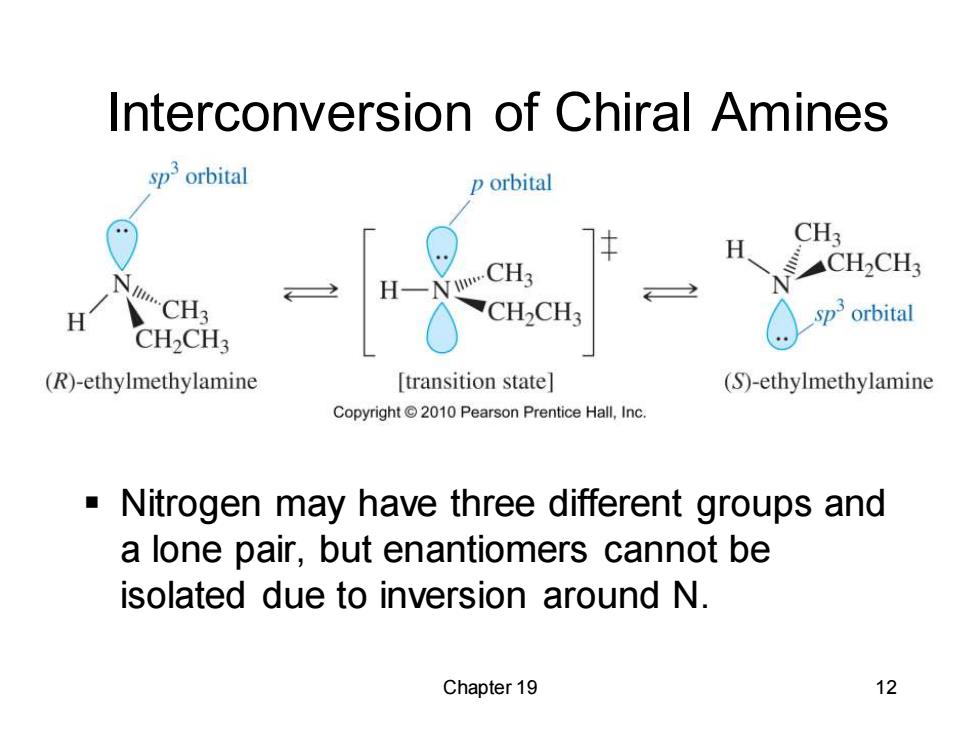
Interconversion of Chiral Amines sp'orbital p orbital H CH3 W.CH3 CH2CH3 H CH2CH3 orbital CH2CH3 (R)-ethylmethylamine [transition state] (S)-ethylmethylamine Copyright2010 Pearson Prentice Hall,Inc. Nitrogen may have three different groups and a lone pair,but enantiomers cannot be isolated due to inversion around N. Chapter 19 12
Chapter 19 12 Interconversion of Chiral Amines ▪ Nitrogen may have three different groups and a lone pair, but enantiomers cannot be isolated due to inversion around N
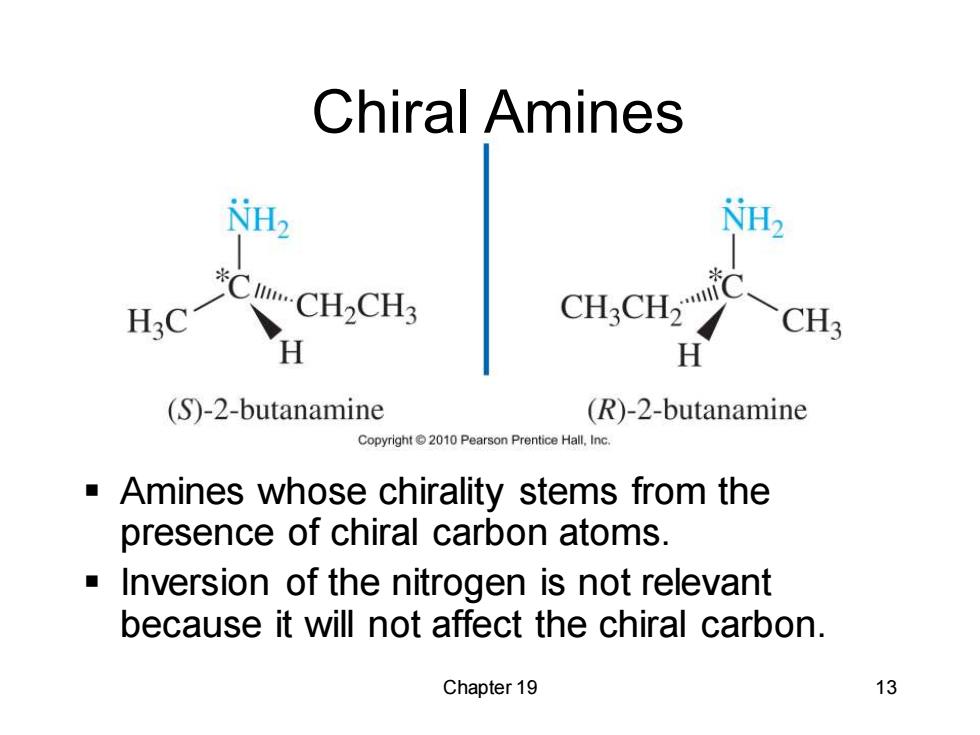
Chiral Amines NH2 NH2 CCH2CH3 H CH.CH. CH3 (S)-2-butanamine (R)-2-butanamine Copyright2010 Pearson Prentice Hall.Inc. Amines whose chirality stems from the presence of chiral carbon atoms. Inversion of the nitrogen is not relevant because it will not affect the chiral carbon. Chapter 19 13
Chapter 19 13 Chiral Amines ▪ Amines whose chirality stems from the presence of chiral carbon atoms. ▪ Inversion of the nitrogen is not relevant because it will not affect the chiral carbon
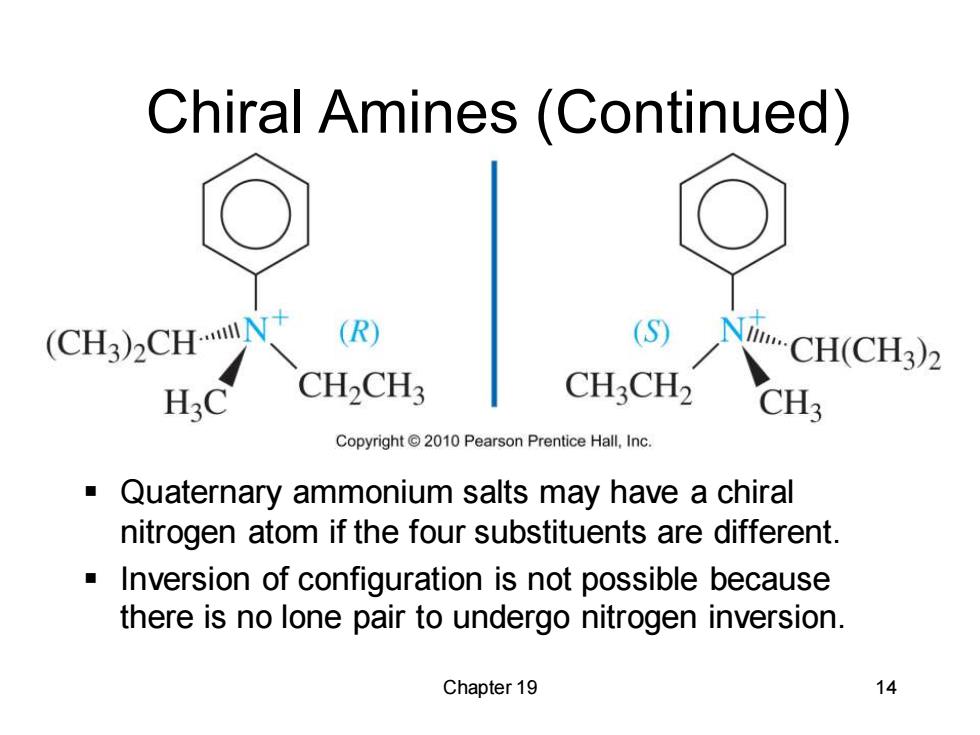
Chiral Amines(Continued) (CH3)2CHN (R) NCH(CH3)2 H3C CH2CH CH3CH2 CH3 Copyright2010 Pearson Prentice Hall,Inc. Quaternary ammonium salts may have a chiral nitrogen atom if the four substituents are different. Inversion of configuration is not possible because there is no lone pair to undergo nitrogen inversion. Chapter 19 14
Chapter 19 14 Chiral Amines (Continued) ▪ Quaternary ammonium salts may have a chiral nitrogen atom if the four substituents are different. ▪ Inversion of configuration is not possible because there is no lone pair to undergo nitrogen inversion
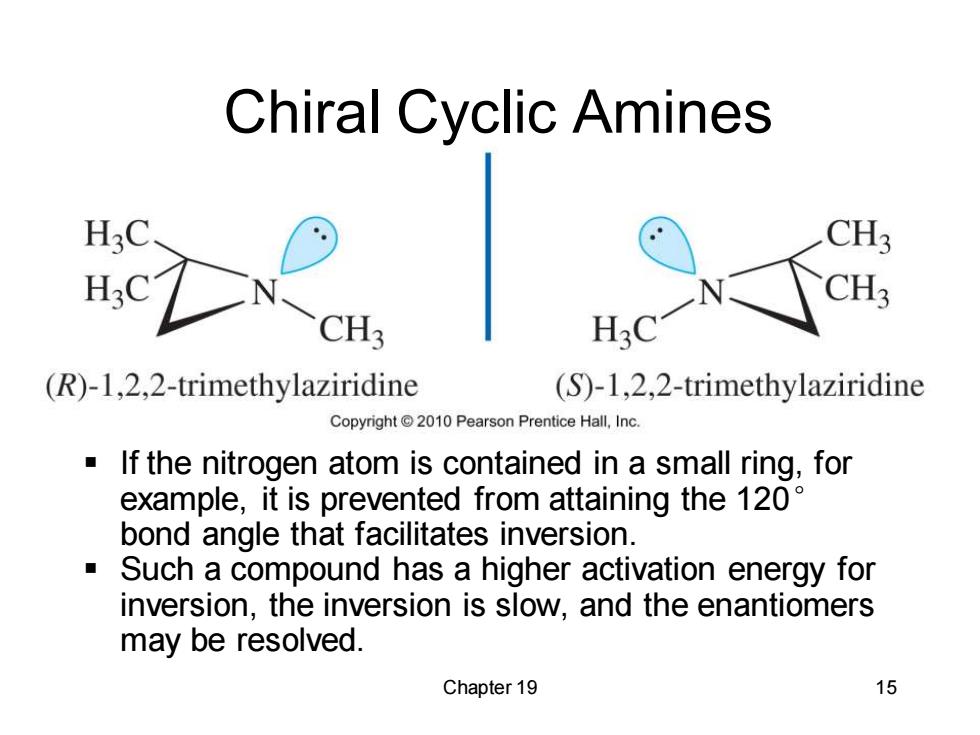
Chiral Cyclic Amines CH3 CH3 CH3 H3C (R)-1,2,2-trimethylaziridine (S)-1,2,2-trimethylaziridine Copyright2010 Pearson Prentice Hall,Inc. If the nitrogen atom is contained in a small ring,for example,it is prevented from attaining the 120 bond angle that facilitates inversion. Such a compound has a higher activation energy for inversion,the inversion is slow,and the enantiomers may be resolved. Chapter 19 15
Chapter 19 15 Chiral Cyclic Amines ▪ If the nitrogen atom is contained in a small ring, for example, it is prevented from attaining the 120° bond angle that facilitates inversion. ▪ Such a compound has a higher activation energy for inversion, the inversion is slow, and the enantiomers may be resolved