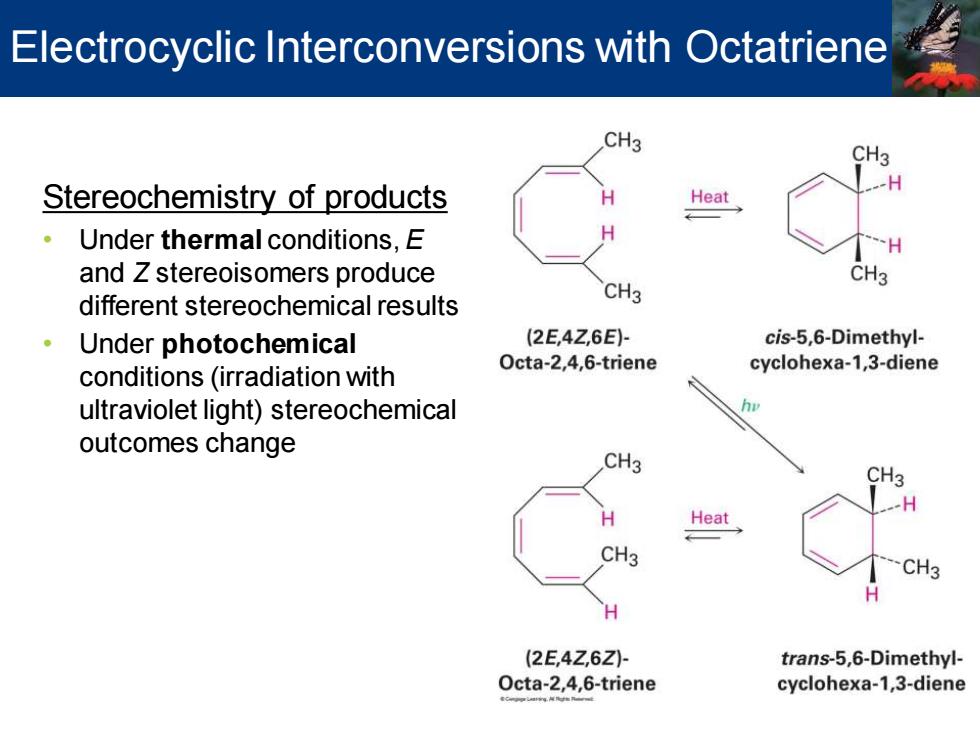
Electrocyclic Interconversions with Octatriene CH3 CH3 Stereochemistry of products Heat Under thermal conditions,E and Z stereoisomers produce CH3 different stereochemical results CH3 Under photochemical (2E,4Z,6E)- cis-5,6-Dimethyl- Octa-2,4,6-triene conditions(irradiation with cyclohexa-1,3-diene ultraviolet light)stereochemical outcomes change CH3 CH3 H Heat CH3 CH3 (2E,4Z,6Z)- trans-5,6-Dimethyl- Octa-2,4,6-triene cyclohexa-1,3-diene
Electrocyclic Interconversions with Octatriene Stereochemistry of products • Under thermal conditions, E and Z stereoisomers produce different stereochemical results • Under photochemical conditions (irradiation with ultraviolet light) stereochemical outcomes change

Electrocyclic Interconversions with Dimethylcyclobutene CH3 CH3 H Heat H CH3 CH3 H cis-3,4-Dimethyl- (2E,4Z)-Hexa-2,4-diene cyclobutene CH3 CH3 Heat CH3 CH3 trans-3,4-Dimethyl- (2E,4E)-Hexa-2,4-diene cyclobutene
Electrocyclic Interconversions with Dimethylcyclobutene

Outermost Lobes of Polyene MOs Must Match to Interact The lobes of like sign can be either on the same side or on opposite sides of the molecule. For a bond to form,the outermost x lobes must rotate so that favorable bonding interaction is achieved positive lobe with positive lobe or negative lobe with negative lobe or Like lobes on same side Like lobes on opposite side Cengags Leaming All Rits Reserved
• The lobes of like sign can be either on the same side or on opposite sides of the molecule. • For a bond to form, the outermost p lobes must rotate so that favorable bonding interaction is achieved • positive lobe with positive lobe or negative lobe with negative lobe Outermost Lobes of Polyene MOs Must Match to Interact
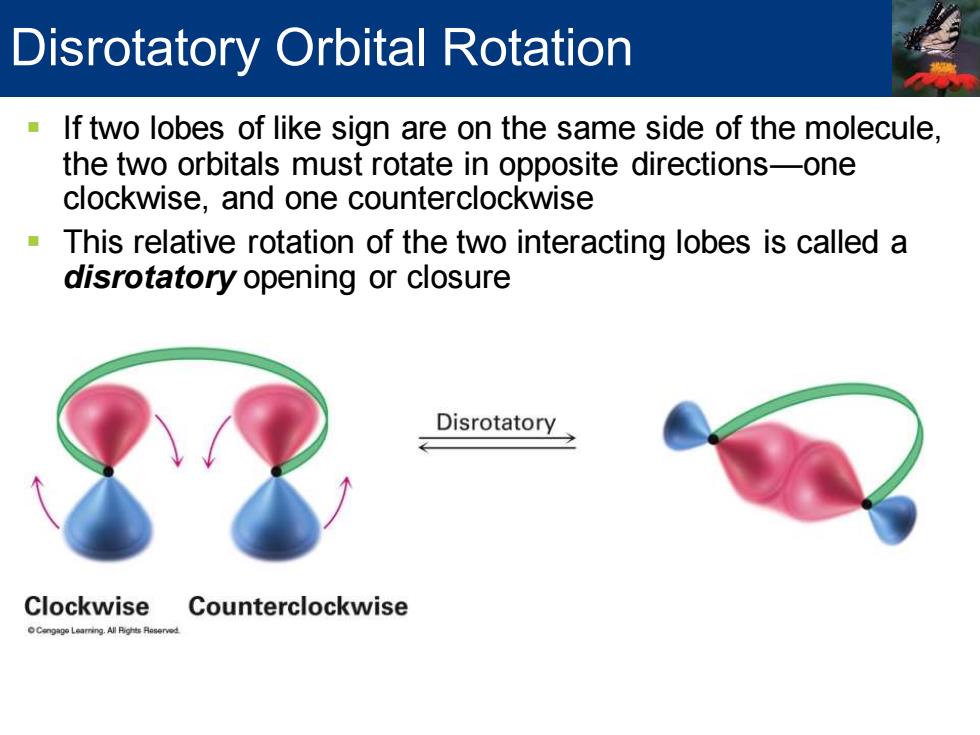
Disrotatory Orbital Rotation If two lobes of like sign are on the same side of the molecule, the two orbitals must rotate in opposite directions-one clockwise,and one counterclockwise This relative rotation of the two interacting lobes is called a disrotatory opening or closure Disrotatory Clockwise Counterclockwise
▪ If two lobes of like sign are on the same side of the molecule, the two orbitals must rotate in opposite directions—one clockwise, and one counterclockwise ▪ This relative rotation of the two interacting lobes is called a disrotatory opening or closure Disrotatory Orbital Rotation
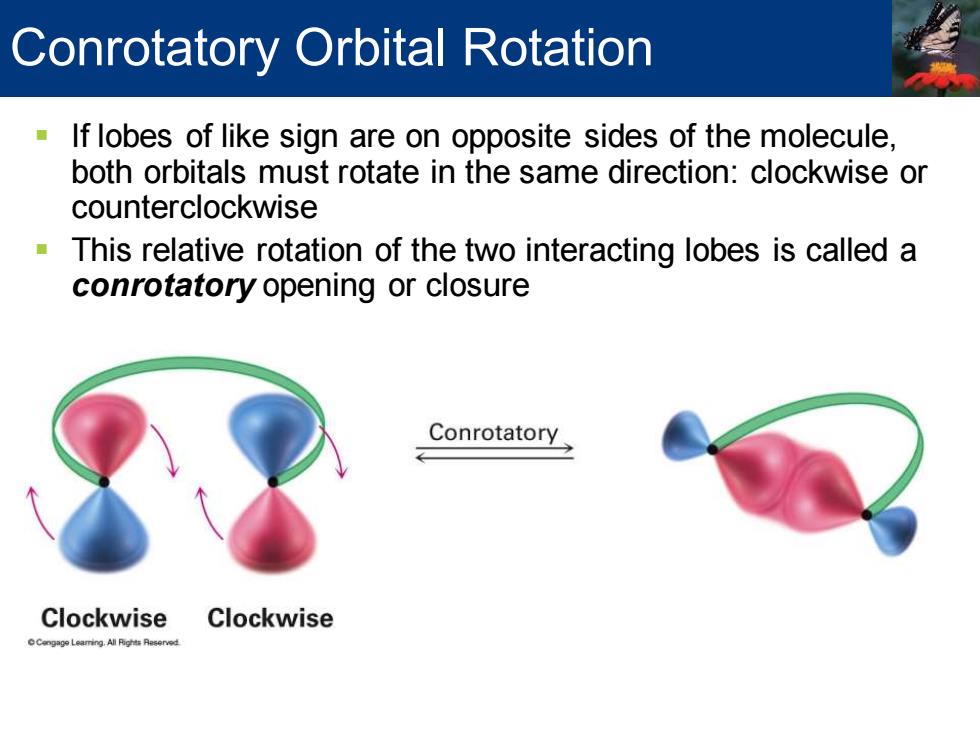
Conrotatory Orbital Rotation If lobes of like sign are on opposite sides of the molecule both orbitals must rotate in the same direction:clockwise or counterclockwise This relative rotation of the two interacting lobes is called a conrotatory opening or closure Conrotatory Clockwise Clockwise
▪ If lobes of like sign are on opposite sides of the molecule, both orbitals must rotate in the same direction: clockwise or counterclockwise ▪ This relative rotation of the two interacting lobes is called a conrotatory opening or closure Conrotatory Orbital Rotation