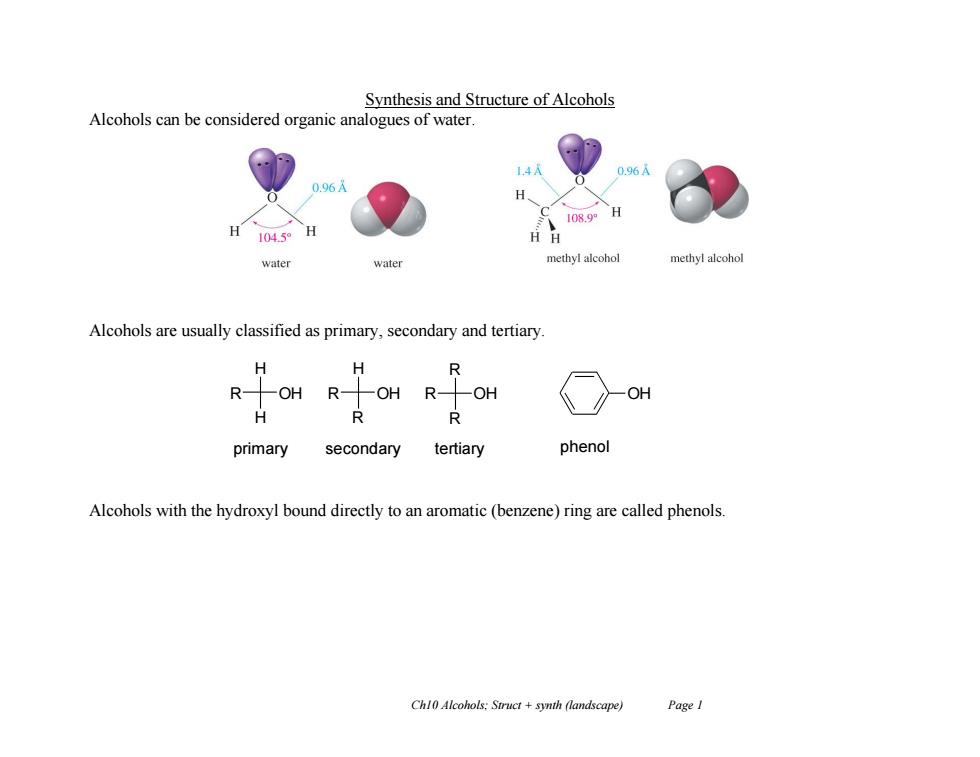
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. 108.9 1045o H HH water methyl alcohol methy!alcohol Alcohols are usually classified as primary,secondary and tertiary. H H R R OH R-OHR-OH OH H 个1 primary secondary tertiary phenol Alcohols with the hydroxyl bound directly to an aromatic (benzene)ring are called phenols Ch10 Alcohols:Struct+synth (landscape) Page 1
Ch10 Alcohols; Struct + synth (landscape) Page 1 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly to an aromatic (benzene) ring are called phenols. R H H OH R H R OH R R R OH primary secondary tertiary OH phenol
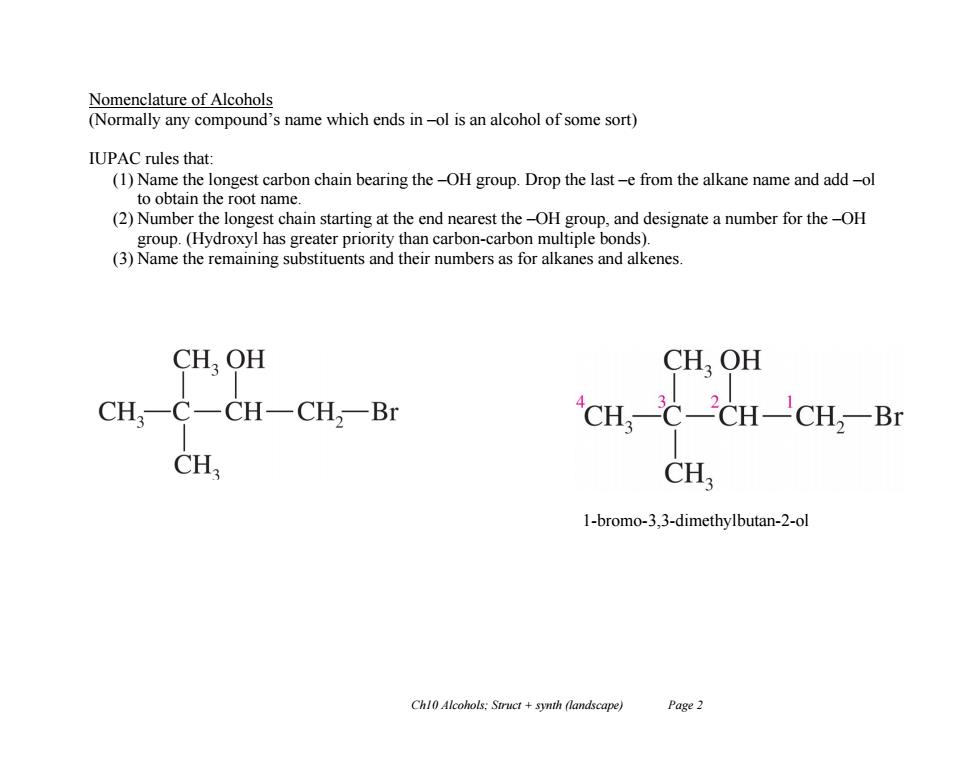
Nomenclature of Alcohols (Normally any compound's name which ends in-ol is an alcohol of some sort) IUPAC rules that: (1)Name the longest carbon chain bearing the-OH group.Drop the last-e from the alkane name and add-ol to obtain the root name. (2)Number the longest chain starting at the end nearest the-OH group,and designate a number for the-OH group.(Hydroxyl has greater priority than carbon-carbon multiple bonds). (3)Name the remaining substituents and their numbers as for alkanes and alkenes CH,OH CHOH CH,-C一CH-CH2-Br CHC-CH-CH:-Br CH CH 1-bromo-3,3-dimethylbutan-2-ol Chl0 Alcohols:Struct synth (landscape) Page 2
Ch10 Alcohols; Struct + synth (landscape) Page 2 Nomenclature of Alcohols (Normally any compound’s name which ends in –ol is an alcohol of some sort) IUPAC rules that: (1) Name the longest carbon chain bearing the –OH group. Drop the last –e from the alkane name and add –ol to obtain the root name. (2) Number the longest chain starting at the end nearest the –OH group, and designate a number for the –OH group. (Hydroxyl has greater priority than carbon-carbon multiple bonds). (3) Name the remaining substituents and their numbers as for alkanes and alkenes. 1-bromo-3,3-dimethylbutan-2-ol
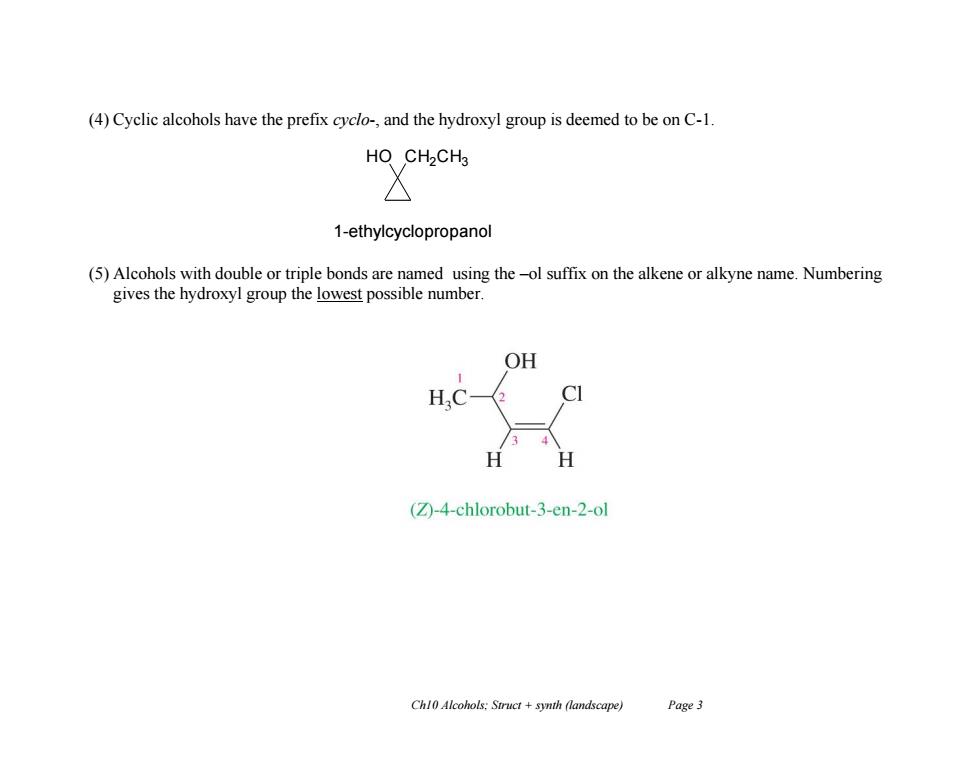
(4)Cyclic alcohols have the prefix cyclo-,and the hydroxyl group is deemed to be on C-1. HO CH2CH3 1-ethylcyclopropanol (5)Alcohols with double or triple bonds are named using the-ol suffix on the alkene or alkyne name.Numbering gives the hydroxyl group the lowest possible number. OH (Z)-4-chlorobut-3-en-2-ol Chl0 Alcohols:Struct synth (landscape) Page 3
Ch10 Alcohols; Struct + synth (landscape) Page 3 (4) Cyclic alcohols have the prefix cyclo-, and the hydroxyl group is deemed to be on C-1. (5) Alcohols with double or triple bonds are named using the –ol suffix on the alkene or alkyne name. Numbering gives the hydroxyl group the lowest possible number. HO CH2CH3 1-ethylcyclopropanol
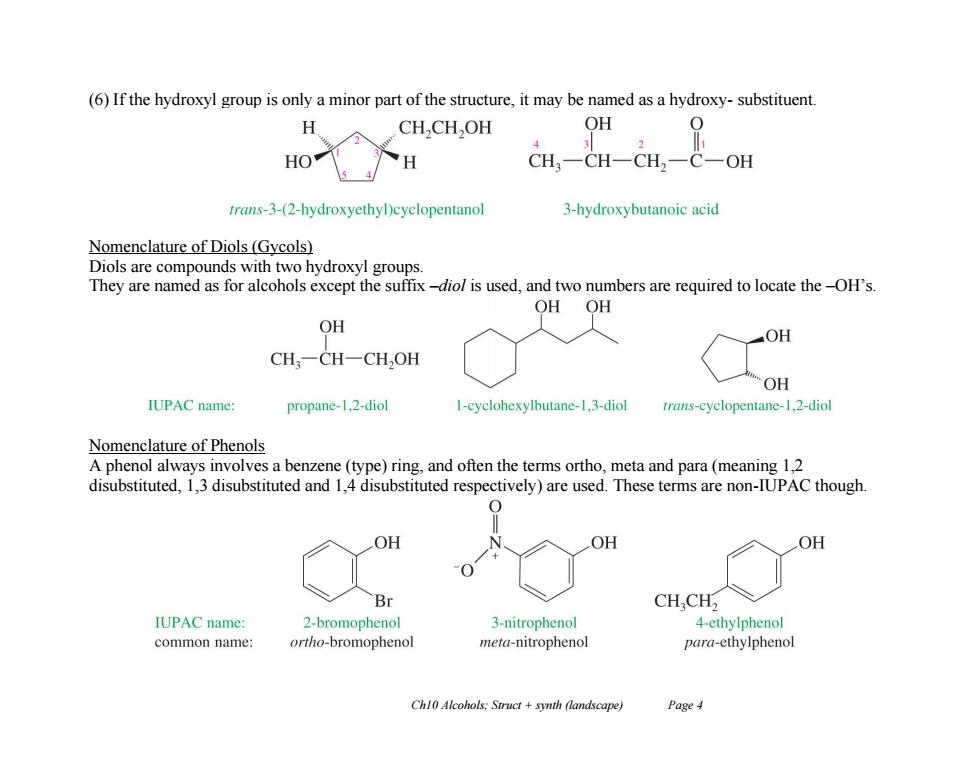
(6)If the hydroxyl group is only a minor part of the structure,it may be named as a hydroxy-substituent. CH,CH,OH OH 0 3 HO H CH一CH一CH2一C一OH trans-3-(2-hydroxyethyl)cyclopentanol 3-hydroxybutanoic acid Nomenclature of Diols (Gycols) Diols are compounds with two hydroxyl groups. They are named as for alcohols except the suffix-diol is used,and two numbers are required to locate the-OH's. OH OH OH OH CH,一CH一CH,OH OH IUPAC name: propane-1.2-diol I-cyclohexylbutane-1.3-diol trans-cyclopentane-1.2-diol Nomenclature of Phenols A phenol always involves a benzene (type)ring,and often the terms ortho,meta and para(meaning 1,2 disubstituted,1,3 disubstituted and 1,4 disubstituted respectively)are used.These terms are non-IUPAC though. 0 OH OH OH Br CH,CH2 IUPAC name: 2-bromophenol 3-nitrophenol 4-ethylphenol common name: ortho-bromophenol meta-nitrophenol para-ethylphenol Chl0 Alcohols:Struct +synth (landscape) Page 4
Ch10 Alcohols; Struct + synth (landscape) Page 4 (6) If the hydroxyl group is only a minor part of the structure, it may be named as a hydroxy- substituent. Nomenclature of Diols (Gycols) Diols are compounds with two hydroxyl groups. They are named as for alcohols except the suffix –diol is used, and two numbers are required to locate the –OH’s. Nomenclature of Phenols A phenol always involves a benzene (type) ring, and often the terms ortho, meta and para (meaning 1,2 disubstituted, 1,3 disubstituted and 1,4 disubstituted respectively) are used. These terms are non-IUPAC though
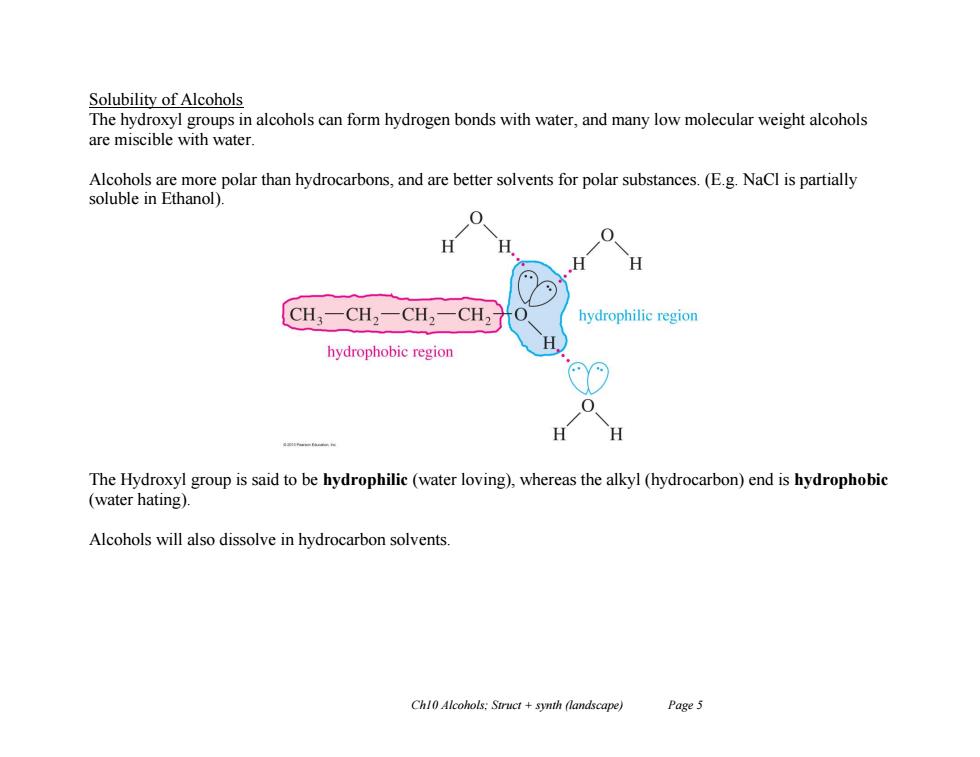
Solubility of Alcohols The hydroxyl groups in alcohols can form hydrogen bonds with water,and many low molecular weight alcohols are miscible with water Alcohols are more polar than hydrocarbons,and are better solvents for polar substances.(E.g.NaCl is partially soluble in Ethanol) H CH3一CH2一CH2一CH2 hydrophilic region hydrophobic region H H The Hydroxyl group is said to be hydrophilic(water loving),whereas the alkyl(hydrocarbon)end is hydrophobic (water hating). Alcohols will also dissolve in hydrocarbon solvents. Chl0 Alcohols:Struct +synth (landscape) Page 5
Ch10 Alcohols; Struct + synth (landscape) Page 5 Solubility of Alcohols The hydroxyl groups in alcohols can form hydrogen bonds with water, and many low molecular weight alcohols are miscible with water. Alcohols are more polar than hydrocarbons, and are better solvents for polar substances. (E.g. NaCl is partially soluble in Ethanol). The Hydroxyl group is said to be hydrophilic (water loving), whereas the alkyl (hydrocarbon) end is hydrophobic (water hating). Alcohols will also dissolve in hydrocarbon solvents