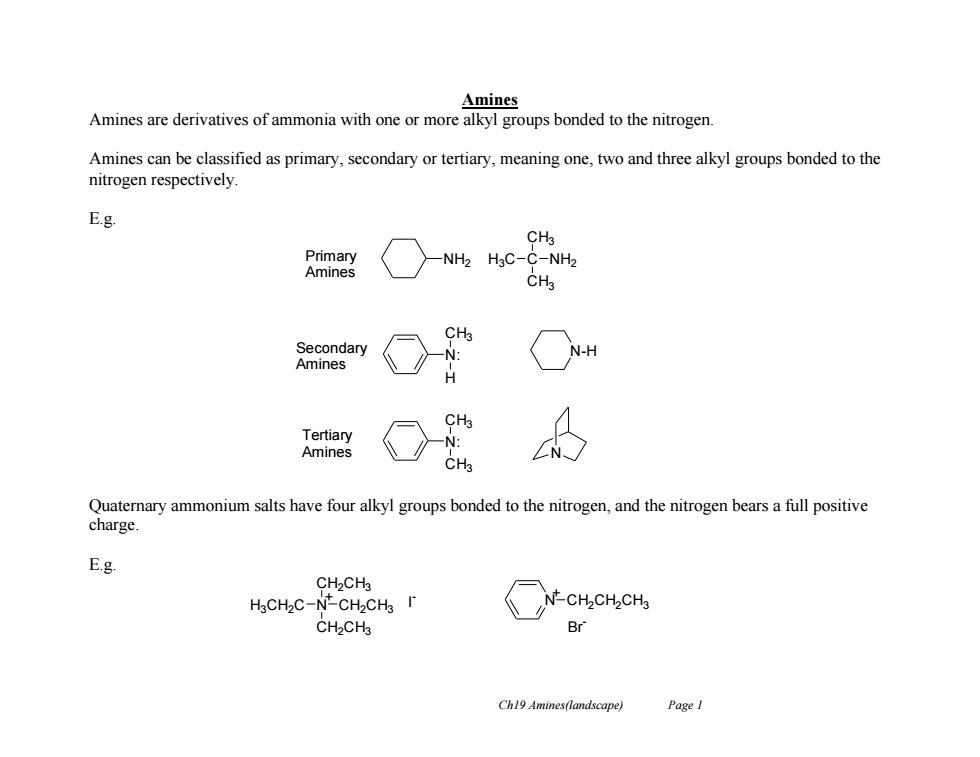
Amines Amines are derivatives of ammonia with one or more alkyl groups bonded to the nitrogen. Amines can be classified as primary,secondary or tertiary,meaning one,two and three alkyl groups bonded to the nitrogen respectively E.g. CH3 Primary -NH2 H3C-C-NH2 Amines CH3 CH3 Secondary N-H Amines Tertiary Amines Quaternary ammonium salts have four alkyl groups bonded to the nitrogen,and the nitrogen bears a full positive charge E.g. CH2CH3 H3CH2C-N-CH2CHs r N-CH2CH2CH3 CH2CHa Br Ch19 Amines(landscape) Page I
Ch19 Amines(landscape) Page 1 Amines Amines are derivatives of ammonia with one or more alkyl groups bonded to the nitrogen. Amines can be classified as primary, secondary or tertiary, meaning one, two and three alkyl groups bonded to the nitrogen respectively. E.g. Quaternary ammonium salts have four alkyl groups bonded to the nitrogen, and the nitrogen bears a full positive charge. E.g. NH2 H3C C CH3 CH3 NH2 Primary Amines Secondary Amines N: H CH3 N-H Tertiary Amines N: CH3 CH3 N H3CH2C N CH2CH3 CH2CH3 CH2CH3 + I - N CH2CH2CH3 + Br -
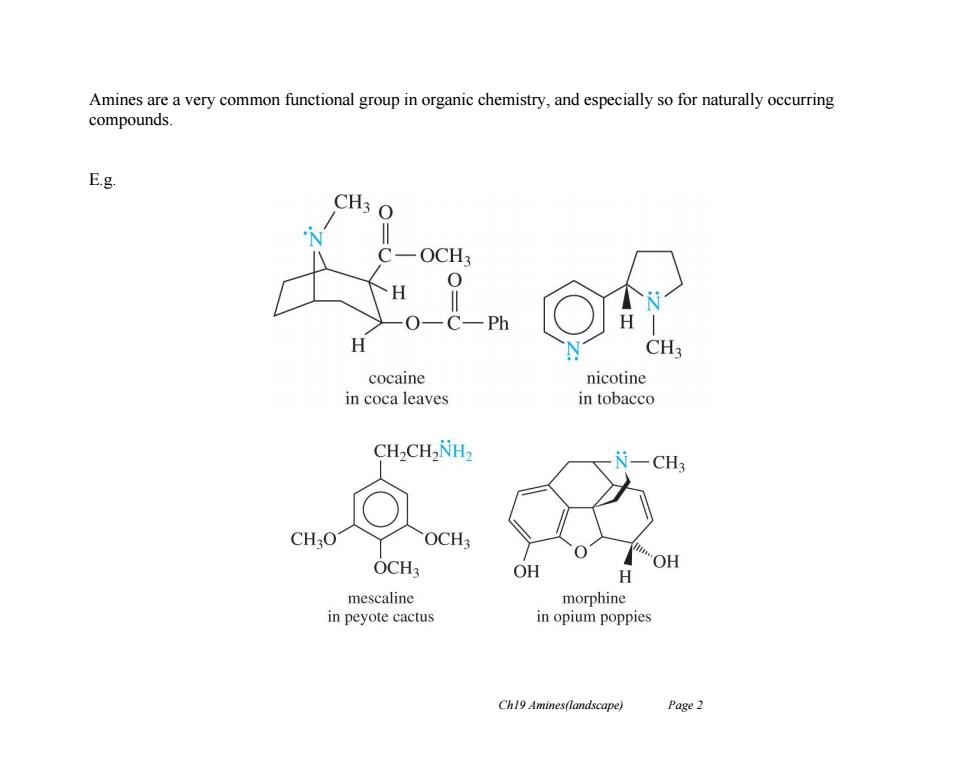
Amines are a very common functional group in organic chemistry,and especially so for naturally occurring compounds. E.g. CH3 O OCH 0 0 Ph H CH3 cocaine nicotine in coca leaves in tobacco CH-CH2NH -CH3 CHO OCH3 OCH3 OH OH H mescaline morphine in peyote cactus in opium poppies Ch19 Amines(landscape) Page 2
Ch19 Amines(landscape) Page 2 Amines are a very common functional group in organic chemistry, and especially so for naturally occurring compounds. E.g
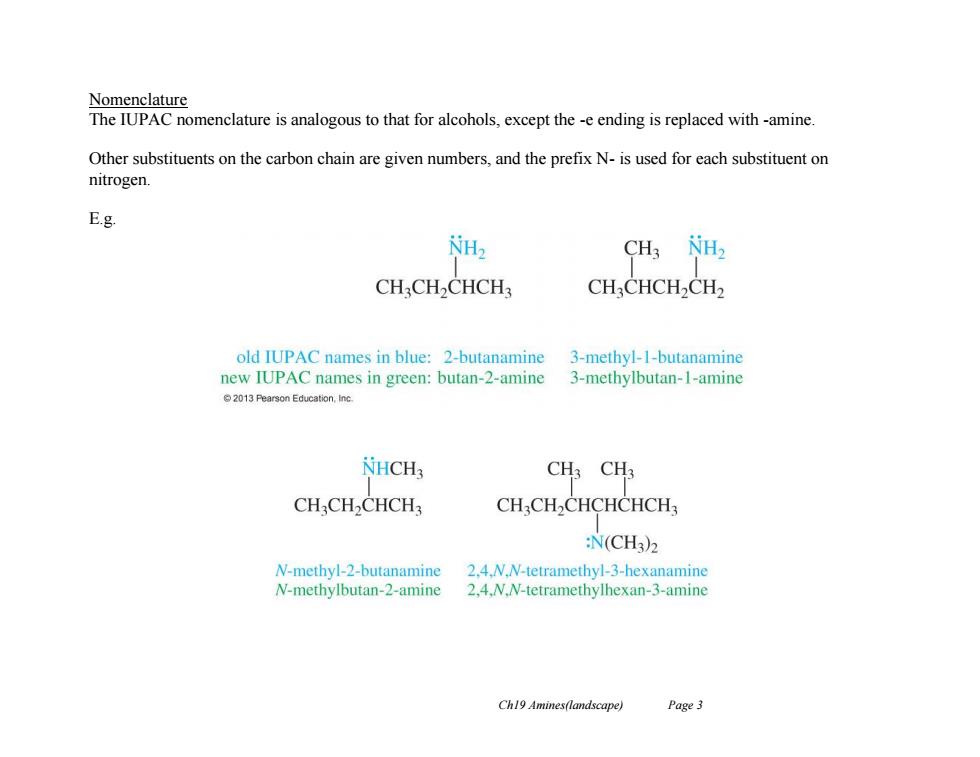
Nomenclature The IUPAC nomenclature is analogous to that for alcohols,except the -e ending is replaced with-amine. Other substituents on the carbon chain are given numbers,and the prefix N-is used for each substituent on nitrogen E.g NH2 CH3 NH2 CHCH2CHCH: CHCHCH2CH2 old IUPAC names in blue:2-butanamine 3-methyl-1-butanamine new IUPAC names in green:butan-2-amine 3-methylbutan-I-amine 2013 Pearson Education.Inc. NHCH3 CH3 CH3 CHCH2CHCH3 CHCH2CHCHCHCH :N(CH3)2 N-methyl-2-butanamine 2.4.N.N-tetramethyl-3-hexanamine N-methylbutan-2-amine 2.4.N.N-tetramethylhexan-3-amine Ch19 Amines(landscape) Page 3
Ch19 Amines(landscape) Page 3 Nomenclature The IUPAC nomenclature is analogous to that for alcohols, except the -e ending is replaced with -amine. Other substituents on the carbon chain are given numbers, and the prefix N- is used for each substituent on nitrogen. E.g
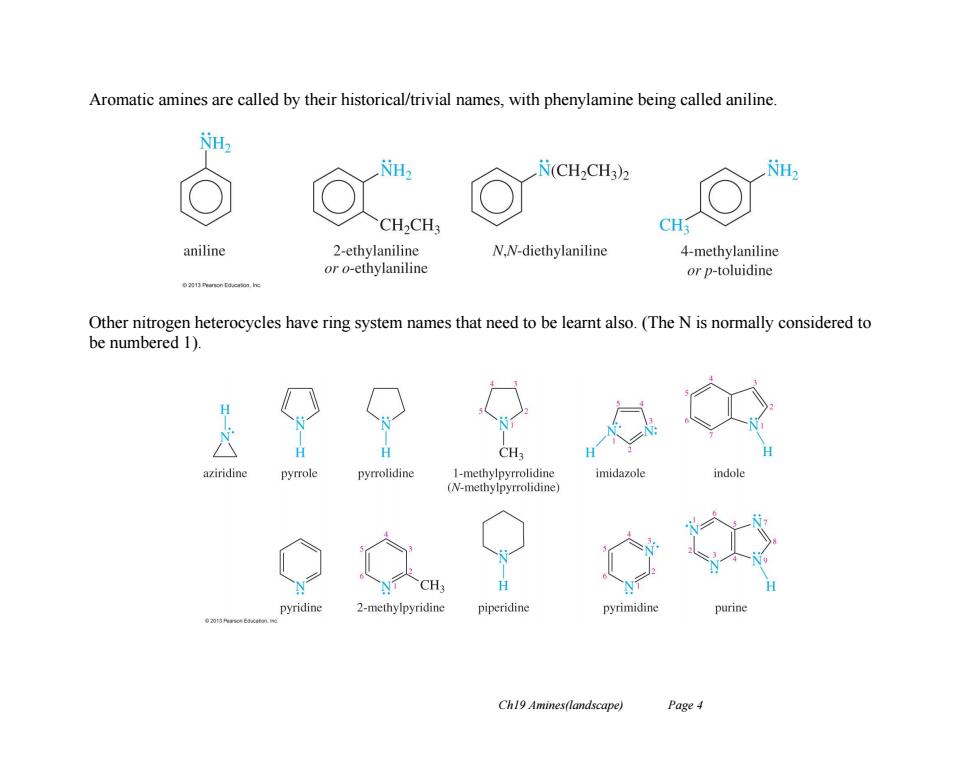
Aromatic amines are called by their historical/trivial names,with phenylamine being called aniline H H N(CH2CH3)2 CH2CH3 CH3 aniline 2-ethylaniline N.N-diethylaniline 4-methylaniline or o-ethylaniline or p-toluidine Other nitrogen heterocycles have ring system names that need to be learnt also.(The N is normally considered to be numbered 1). CH3 pyrrole pyrrolidine 1-methylpyrrolidine imidazole ndo (N-methylpyrrolidine) CH3 pyridine 2-methylpyridine piperidine pyrimidine Ch19 Amines(landscape) Page 4
Ch19 Amines(landscape) Page 4 Aromatic amines are called by their historical/trivial names, with phenylamine being called aniline. Other nitrogen heterocycles have ring system names that need to be learnt also. (The N is normally considered to be numbered 1)
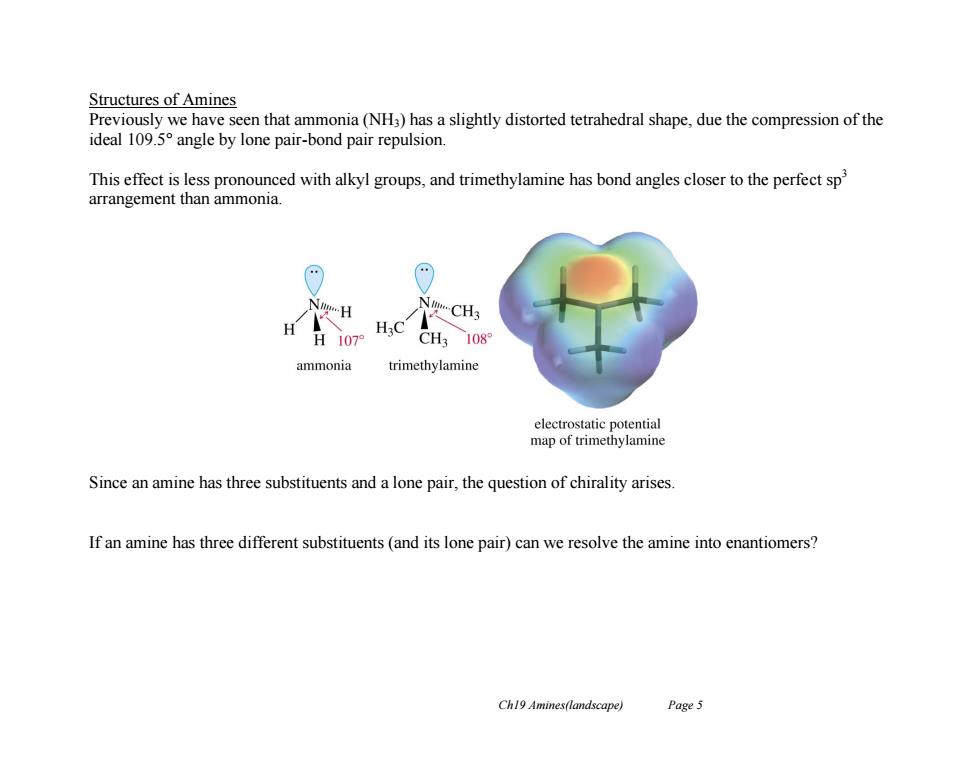
Structures of Amines Previously we have seen that ammonia(NH3)has a slightly distorted tetrahedral shape,due the compression of the ideal 109.5 angle by lone pair-bond pair repulsion. This effect is less pronounced with alkyl groups,and trimethylamine has bond angles closer to the perfect sp arrangement than ammonia. NgH N-CH3 H107 CH3 108 ammonia trimethylamine electrostatic potential map of trimethylamine Since an amine has three substituents and a lone pair,the question of chirality arises. If an amine has three different substituents(and its lone pair)can we resolve the amine into enantiomers? Ch19 Amines(landscape) Page 5
Ch19 Amines(landscape) Page 5 Structures of Amines Previously we have seen that ammonia (NH3) has a slightly distorted tetrahedral shape, due the compression of the ideal 109.5° angle by lone pair-bond pair repulsion. This effect is less pronounced with alkyl groups, and trimethylamine has bond angles closer to the perfect sp3 arrangement than ammonia. Since an amine has three substituents and a lone pair, the question of chirality arises. If an amine has three different substituents (and its lone pair) can we resolve the amine into enantiomers?