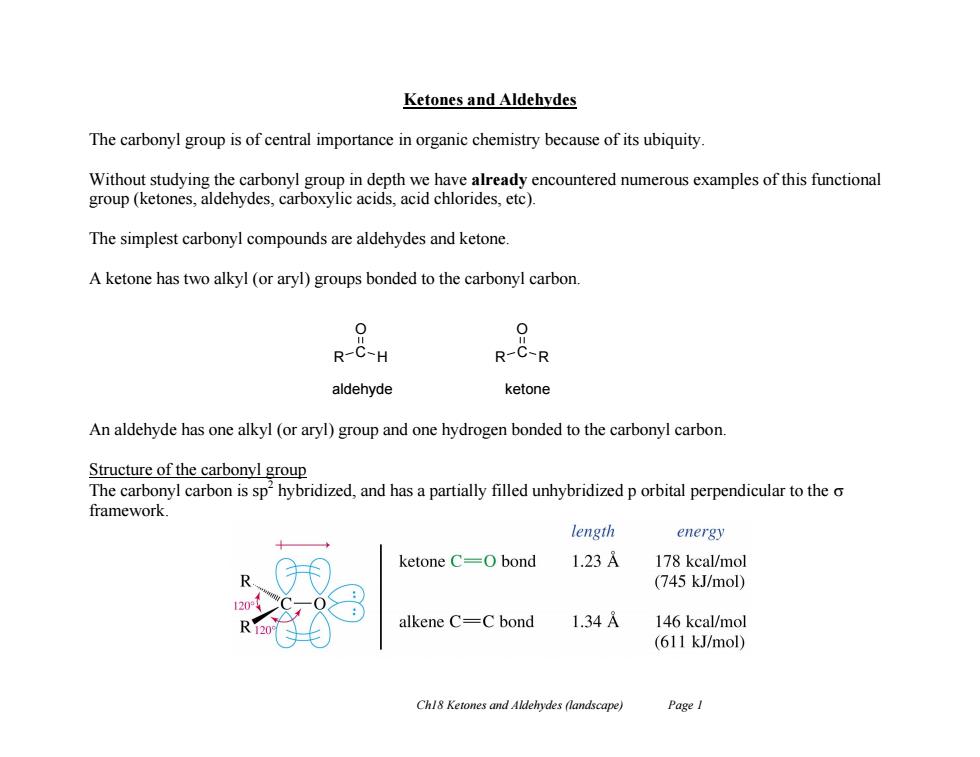
Ketones and Aldehydes The carbonyl group is of central importance in organic chemistry because of its ubiquity Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones,aldehydes,carboxylic acids,acid chlorides.etc). The simplest carbonyl compounds are aldehydes and ketone. A ketone has two alkyl (or aryl)groups bonded to the carbonyl carbon. 0 R-C-H R-C-R aldehyde ketone An aldehyde has one alkyl (or aryl)group and one hydrogen bonded to the carbonyl carbon. Structure of the carbonyl group The carbonyl carbon is sp'hybridized,and has a partially filled unhybridized p orbital perpendicular to the o framework. length energy ketone C=0 bond 1.23A 178 kcal/mol (745 kJ/mol) alkene C=C bond 1.34 146 kcal/mol (611 kJ/mol) Chl8 Ketones and Aldehydes (landscape) Page I
Ch18 Ketones and Aldehydes (landscape) Page 1 Ketones and Aldehydes The carbonyl group is of central importance in organic chemistry because of its ubiquity. Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The simplest carbonyl compounds are aldehydes and ketone. A ketone has two alkyl (or aryl) groups bonded to the carbonyl carbon. An aldehyde has one alkyl (or aryl) group and one hydrogen bonded to the carbonyl carbon. Structure of the carbonyl group The carbonyl carbon is sp2 hybridized, and has a partially filled unhybridized p orbital perpendicular to the framework. R C H O R C R O aldehyde ketone
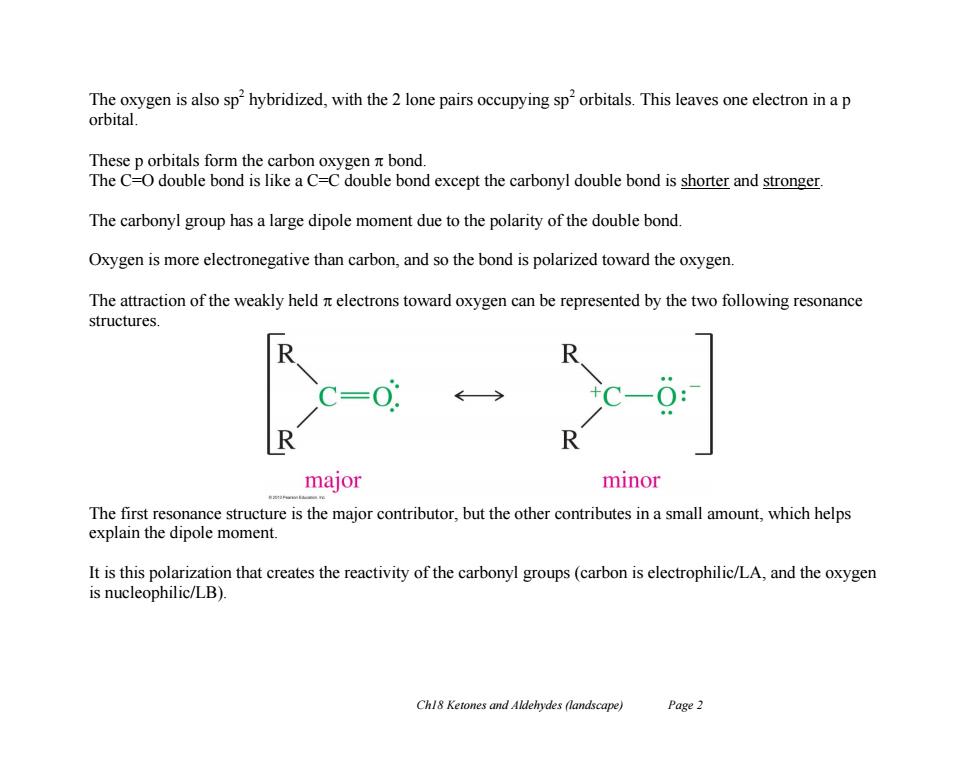
The oxygen is also sp hybridized,with the 2 lone pairs occupying sp'orbitals.This leaves one electron in a p orbital. These p orbitals form the carbon oxygen bond. The C=O double bond is like a C=C double bond except the carbonyl double bond is shorter and stronger. The carbonyl group has a large dipole moment due to the polarity of the double bond. Oxygen is more electronegative than carbon,and so the bond is polarized toward the oxygen. The attraction of the weakly held nt electrons toward oxygen can be represented by the two following resonance structures. R major minor The first resonance structure is the major contributor,but the other contributes in a small amount,which helps explain the dipole moment. It is this polarization that creates the reactivity of the carbonyl groups(carbon is electrophilic/LA,and the oxygen is nucleophilic/LB). Chl8 Ketones and Aldehydes (landscape) Page 2
Ch18 Ketones and Aldehydes (landscape) Page 2 The oxygen is also sp2 hybridized, with the 2 lone pairs occupying sp2 orbitals. This leaves one electron in a p orbital. These p orbitals form the carbon oxygen bond. The C=O double bond is like a C=C double bond except the carbonyl double bond is shorter and stronger. The carbonyl group has a large dipole moment due to the polarity of the double bond. Oxygen is more electronegative than carbon, and so the bond is polarized toward the oxygen. The attraction of the weakly held electrons toward oxygen can be represented by the two following resonance structures. The first resonance structure is the major contributor, but the other contributes in a small amount, which helps explain the dipole moment. It is this polarization that creates the reactivity of the carbonyl groups (carbon is electrophilic/LA, and the oxygen is nucleophilic/LB)
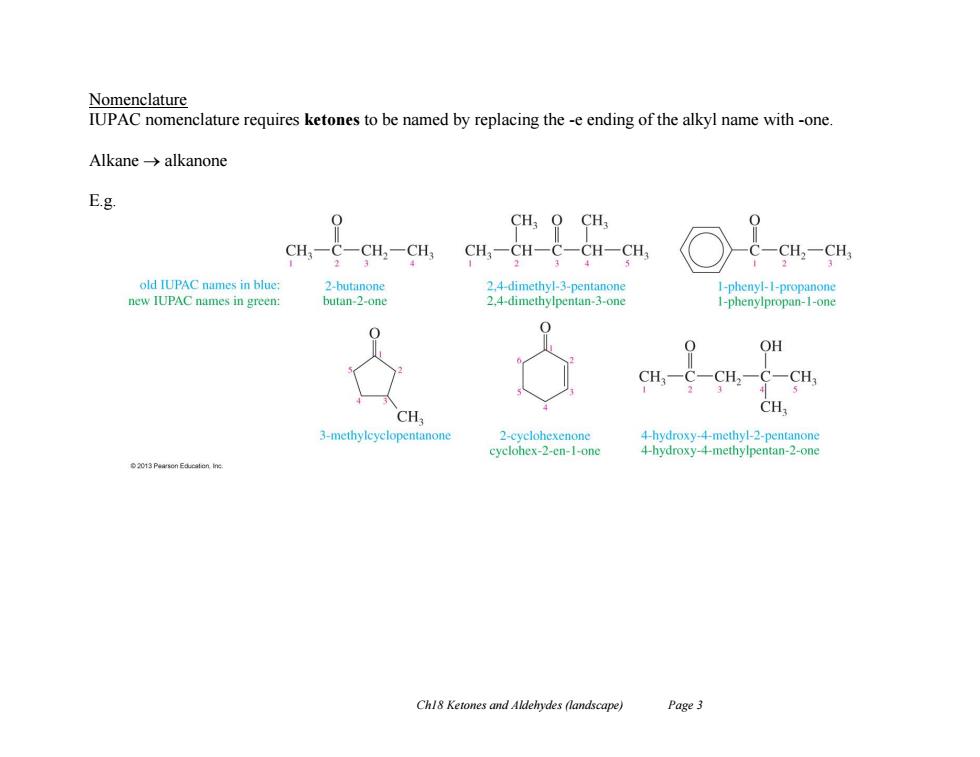
Nomenclature IUPAC nomenclature requires ketones to be named by replacing the -e ending of the alkyl name with -one Alkane-→alkanone Eg CH;O CH CH- -CH2一CH CH-CH-C-CH-CH CH2一CH 1 old IUPAC names in blue: 2-butanone 2.4-dimethyl-3-pentanone 1-phenyl-1-propanone new IUPAC names in green: butan-2-one 2.4-dimethylpentan-3-one 1-phenylpropan-1-one 0 OH CH3一C-CH2一C一CH CH CH 3-methylcyclopentanone 2-cyclohexenone 4-hydroxy-4-methyl-2-pentanone cyclohex-2-en-1-one 4-hydroxy-4-methylpentan-2-one Chl8 Ketones and Aldehydes (landscape) Page3
Ch18 Ketones and Aldehydes (landscape) Page 3 Nomenclature IUPAC nomenclature requires ketones to be named by replacing the -e ending of the alkyl name with -one. Alkane alkanone E.g
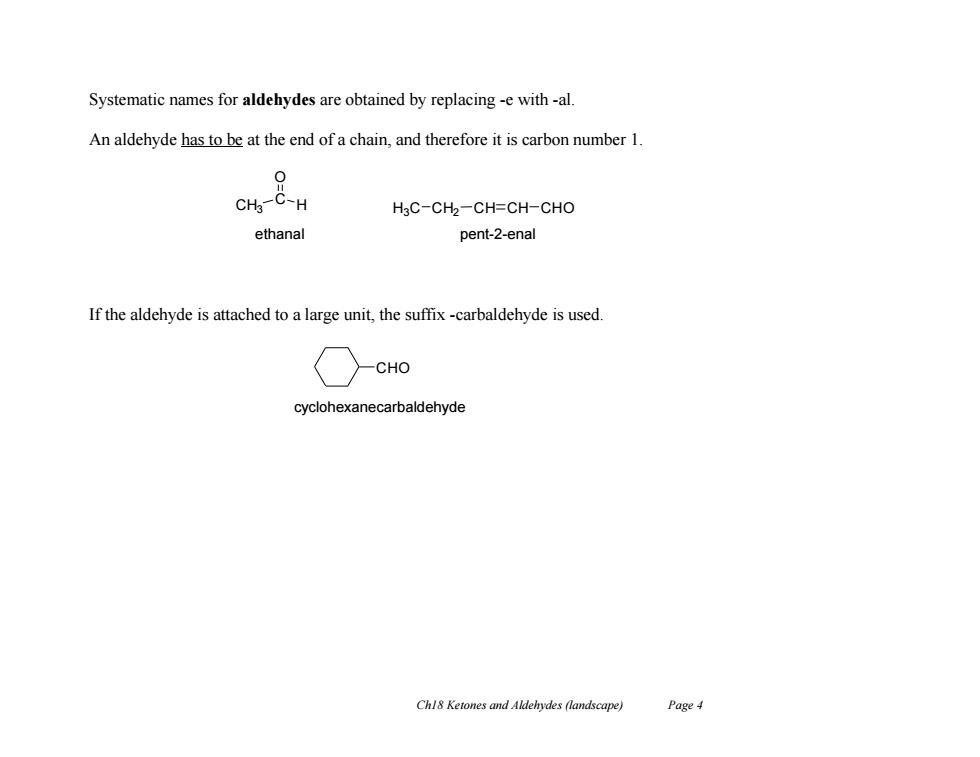
Systematic names for aldehydes are obtained by replacing -e with-al. An aldehyde has to be at the end of a chain,and therefore it is carbon number 1. 0 CHg-C-H H3C-CH2-CH=CH-CHO ethanal pent-2-enal If the aldehyde is attached to a large unit,the suffix-carbaldehyde is used. CHO cyclohexanecarbaldehyde Chl8 Ketones and Aldehydes(landscape) Page 4
Ch18 Ketones and Aldehydes (landscape) Page 4 Systematic names for aldehydes are obtained by replacing -e with -al. An aldehyde has to be at the end of a chain, and therefore it is carbon number 1. If the aldehyde is attached to a large unit, the suffix -carbaldehyde is used. CH3 C H O H3C CH2 CH CH CHO ethanal pent-2-enal CHO cyclohexanecarbaldehyde
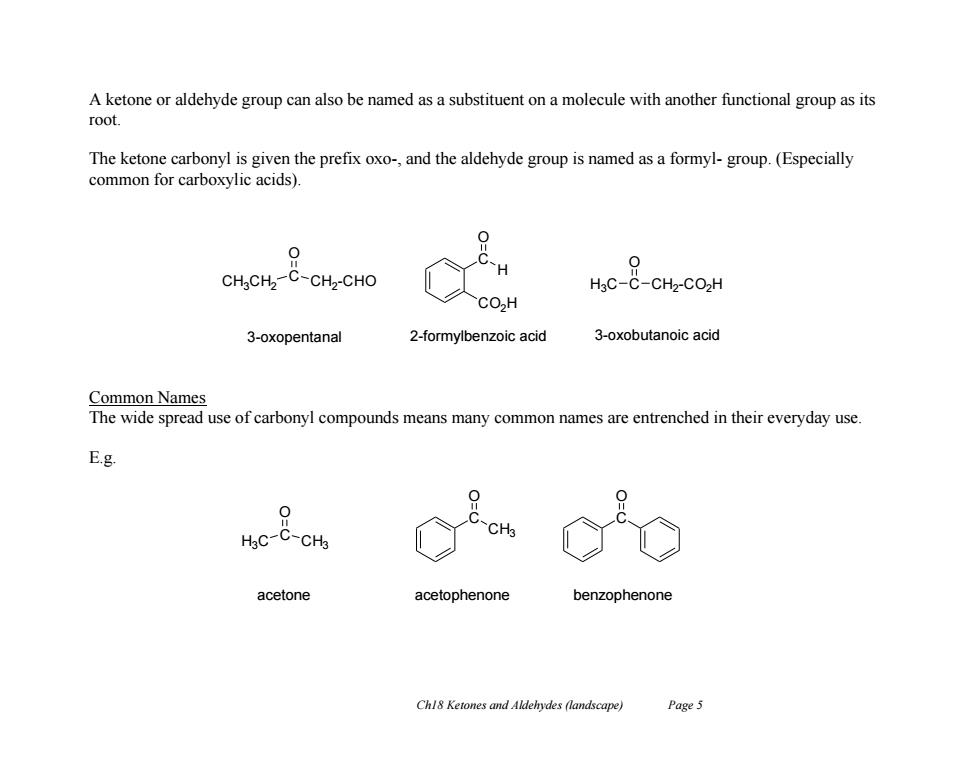
A ketone or aldehyde group can also be named as a substituent on a molecule with another functional group as its root. The ketone carbonyl is given the prefix oxo-,and the aldehyde group is named as a formyl-group.(Especially common for carboxylic acids). 0 CH CH2-C-CH2-CHO HgC-C-CH2-CO2H CO,H 3-oxopentanal 2-formylbenzoic acid 3-oxobutanoic acid Common Names The wide spread use of carbonyl compounds means many common names are entrenched in their everyday use. E.g. 0 HgC-C-CH acetone acetophenone benzophenone Chl8 Ketones and Aldehydes (landscape) Page 5
Ch18 Ketones and Aldehydes (landscape) Page 5 A ketone or aldehyde group can also be named as a substituent on a molecule with another functional group as its root. The ketone carbonyl is given the prefix oxo-, and the aldehyde group is named as a formyl- group. (Especially common for carboxylic acids). Common Names The wide spread use of carbonyl compounds means many common names are entrenched in their everyday use. E.g. CH3CH2 C CH2 -CHO O 3-oxopentanal C CO2H O H H3C C O CH2 -CO2H 2-formylbenzoic acid 3-oxobutanoic acid H3C C CH3 O acetone C O CH3 acetophenone C O benzophenone