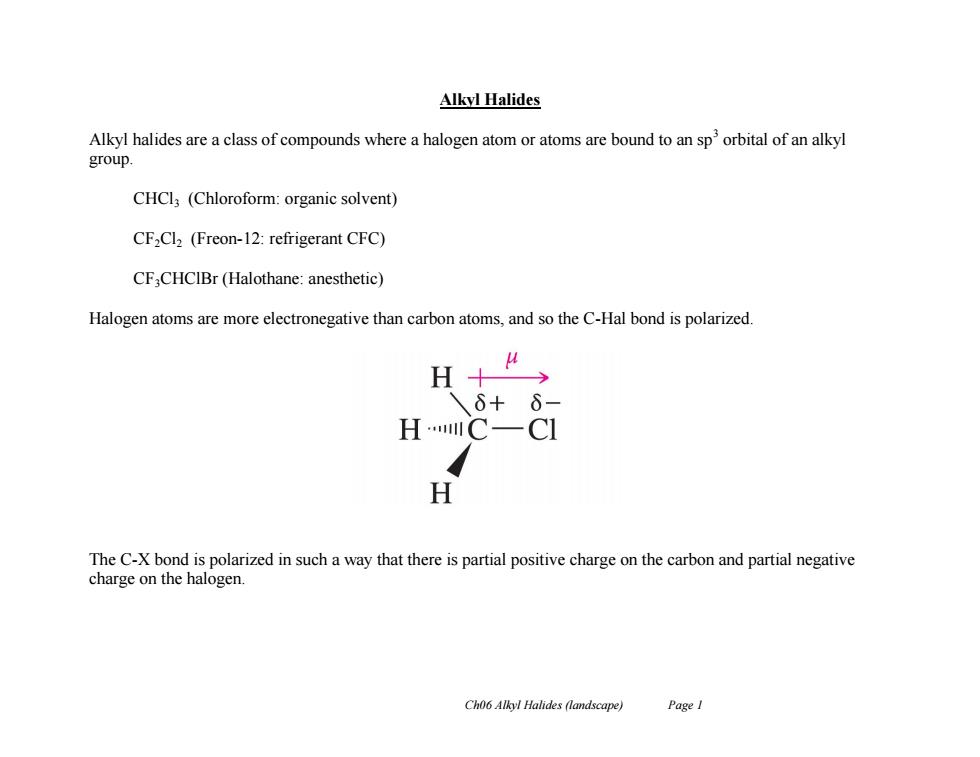
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp'orbital of an alkyl group. CHCl (Chloroform:organic solvent) CF,Cl2 (Freon-12:refrigerant CFC) CF,CHCIBr(Halothane:anesthetic) Halogen atoms are more electronegative than carbon atoms,and so the C-Hal bond is polarized. A 6+6 HC一C H The C-X bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen. Ch06 Alkyl Halides (landscape) Page I
Ch06 Alkyl Halides (landscape) Page 1 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp3 orbital of an alkyl group. CHCl3 (Chloroform: organic solvent) CF2Cl2 (Freon-12: refrigerant CFC) CF3CHClBr (Halothane: anesthetic) Halogen atoms are more electronegative than carbon atoms, and so the C-Hal bond is polarized. The C-X bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen
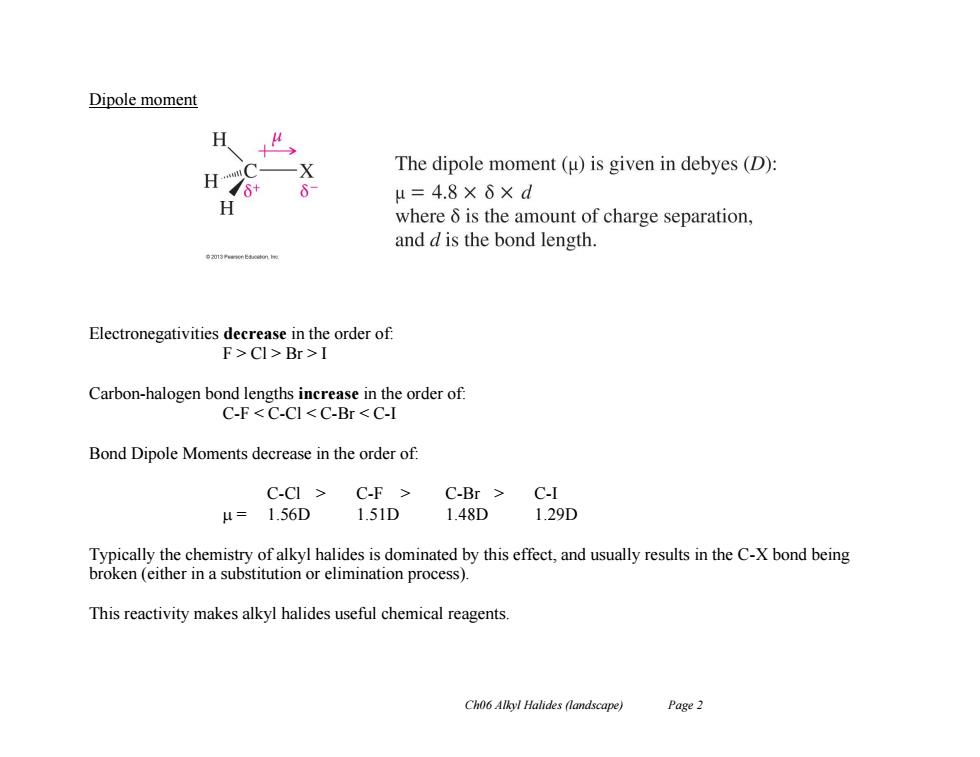
Dipole moment 夕 The dipole moment(u)is given in debyes(D): H7* μ=4.8×6×d where 6 is the amount of charge separation, and d is the bond length. Electronegativities decrease in the order of: F>Cl>Br>I Carbon-halogen bond lengths increase in the order of: C-F<C-CI<C-Br<C-I Bond Dipole Moments decrease in the order of: C-CI C-F C-Br C-I μ=1.56D 1.51D1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect,and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents Ch06 Alkyl Halides (landscape) Page 2
Ch06 Alkyl Halides (landscape) Page 2 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents

Nomenclature According to IUPAC,alkyl halides are treated as alkanes with a halogen substituent. The halogen prefixes are Fluoro-,Chloro-,Bromo-and lodo-. Examples: F-CH2CH3 H 、CHs ci H fluoroethane trans-1-chloro-3-methylcyclopentane Often compounds of CH2X2 type are called methylene halides.(CH2Cl2 is methylene chloride). CHX,type compounds are called haloforms.(CHI,is iodoform). CX type compounds are called carbon tetrahalides.(CF is carbon tetrafluoride). Alkyl halides can be primary (1),secondary(2)or tertiary (3). H R R-X R- -X R- H H R 10 20 Other types: A geminal(gem)dihalide has two halogens on the same carbon. A vicinal dihalide has halogens on adjacent carbon atoms. BrBr CI CI RR R-R RR gem-dibromide vicinal dichloride Ch06 Alkyl Halides (landscape) Page 3
Ch06 Alkyl Halides (landscape) Page 3 Nomenclature According to IUPAC, alkyl halides are treated as alkanes with a halogen substituent. The halogen prefixes are Fluoro-, Chloro-, Bromo- and Iodo-. Examples: Often compounds of CH2X2 type are called methylene halides. (CH2Cl2 is methylene chloride). CHX3 type compounds are called haloforms. (CHI3 is iodoform). CX4 type compounds are called carbon tetrahalides. (CF4 is carbon tetrafluoride). Alkyl halides can be primary (1°), secondary (2°) or tertiary (3°). Other types: A geminal (gem) dihalide has two halogens on the same carbon. A vicinal dihalide has halogens on adjacent carbon atoms. F CH2CH3 Cl H H CH3 fluoroethane trans-1-chloro-3-methylcyclopentane R H H X 1 o R R H X 2 o R R R X 3 o R Br Br R gem-dibromide R R Cl R Cl R vicinal dichloride

Preparation of Alkyl Halides Numerous ways to make alkyl halides (la)Free Radical Halogenation Usually this method gives mixtures of mono-,di-,tri-etc halogenated compounds,which is considered an inefficient method for the synthesis of a desired compound. Consider propane: 入+ch2hvCH-Ct-C比Cl+_CHgCHCL-CHa CH3-CH2-CHCl2 +CH3-CCl2-CH3 and others Sometimes if there can be control over the selectivity of halogenation this is a useful route. 以 Cl hv Chlorocyclohexane (50%) CH3 CH3 H3C- H +Br2 hv H3C Br CH3 CH3 t-Butylbromide (90%) Ch06 Alkyl Halides (landscape) Page 4
Ch06 Alkyl Halides (landscape) Page 4 Preparation of Alkyl Halides Numerous ways to make alkyl halides. (1a) Free Radical Halogenation Usually this method gives mixtures of mono-, di-, tri- etc halogenated compounds, which is considered an inefficient method for the synthesis of a desired compound. Consider propane: Sometimes if there can be control over the selectivity of halogenation this is a useful route. + Cl2 h CH3 -CH2 -CH2Cl + CH3 -CHCl-CH3 CH3 -CH2 -CHCl2 +CH3 -CCl2 -CH3 and others H Cl + Cl2 h Chlorocyclohexane (50%) H H CH3 H3C CH3 H + Br2 h CH3 H3C CH3 Br t-Butylbromide (90%)
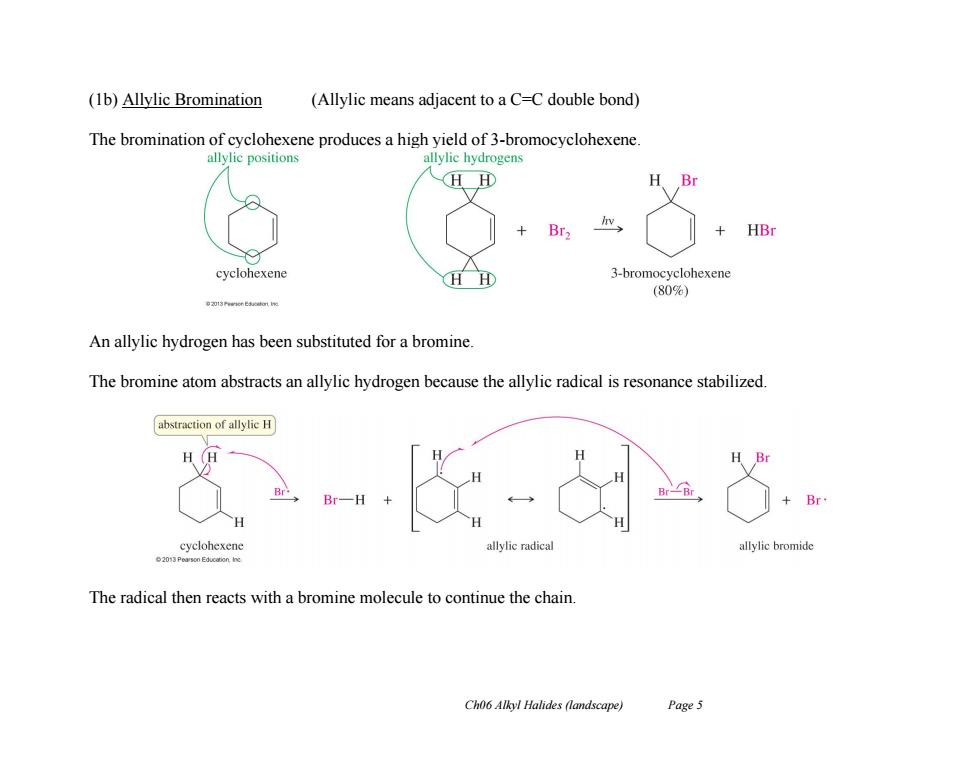
(1b)Allylic Bromination (Allylic means adjacent to a C=C double bond) The bromination of cyclohexene produces a high yield of 3-bromocyclohexene. allylie positions allylic hydrogens HH H Br HBr cyclohexene 3-bromocyclohexene (80%) An allylic hydrogen has been substituted for a bromine. The bromine atom abstracts an allylic hydrogen because the allylic radical is resonance stabilized abstraction of allylic H cyclohexene allylic radica allylic bromide 620 The radical then reacts with a bromine molecule to continue the chain. Ch06 Alkyl Halides (landscape) Page 5
Ch06 Alkyl Halides (landscape) Page 5 (1b) Allylic Bromination (Allylic means adjacent to a C=C double bond) The bromination of cyclohexene produces a high yield of 3-bromocyclohexene. An allylic hydrogen has been substituted for a bromine. The bromine atom abstracts an allylic hydrogen because the allylic radical is resonance stabilized. The radical then reacts with a bromine molecule to continue the chain