
Alkynes Alkynes or acetylenes are compounds that contain a carbon-carbon triple bond. E.g. H-C=C-H CHgCH2-C=C-H HgC-C=C-CH3 acetylene The triple bond results in a molecular formula of C H2n2 Ethane CH 0 elements of unsaturation Ethene CH 1 element of unsaturation Ethyne C2H2 2 elements of unsaturation The triple bond contributes two elements of unsaturation. Nomenclature of Alkynes IUPAC nomenclature is similar to that for alkenes,except the-ane ending is replaced with-yne. The chain is numbered from the end closest to the triple bond. CH, Br H-C=C-H CH,-C=C-H CH,-C=C-CH,CH,-CH-C=C-CH,-CH-CH, old IUPAC name: ethyne propyne 2-butyne 6-bromo-2-methyl-3-heptyne new IUPAC name: ethyne propyne but-2-yne 6-bromo-2-methylhept-3-yne Ch09 Alkynes (landscape) Page I
Ch09 Alkynes (landscape) Page 1 Alkynes Alkynes or acetylenes are compounds that contain a carbon–carbon triple bond. E.g. The triple bond results in a molecular formula of CnH2n-2 Ethane C2H6 0 elements of unsaturation Ethene C2H4 1 element of unsaturation Ethyne C2H2 2 elements of unsaturation The triple bond contributes two elements of unsaturation. Nomenclature of Alkynes IUPAC nomenclature is similar to that for alkenes, except the –ane ending is replaced with –yne. The chain is numbered from the end closest to the triple bond. H C C acetylene H CH3CH2 C C H H3C C C CH3
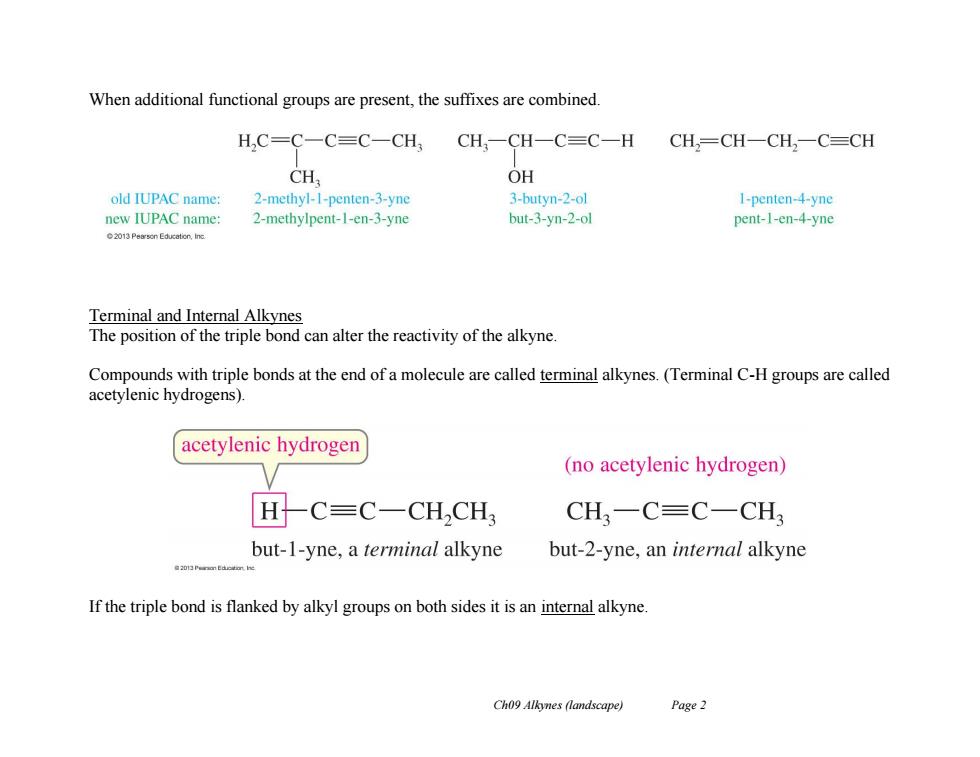
When additional functional groups are present,the suffixes are combined. H,C-C-C=C-CH, CH,一CH-C=C-H CH,=CH一CH2一C=CH CH, OH old IUPAC name: 2-methyl-I-penten-3-yne 3-butyn-2-ol 1-penten-4-yne new IUPAC name: 2-methylpent-1-en-3-yne but-3-yn-2-ol pent-1-en-4-yne 2013 Pearson Education,Inc Terminal and Internal Alkynes The position of the triple bond can alter the reactivity of the alkyne. Compounds with triple bonds at the end of a molecule are called terminal alkynes.(Terminal C-H groups are called acetylenic hydrogens). acetylenic hydrogen (no acetylenic hydrogen) HC=C一CH,CH CH3一C=C一CH3 but-1-yne,a terminal alkyne but-2-yne,an internal alkyne If the triple bond is flanked by alkyl groups on both sides it is an internal alkyne. Ch09 Alkynes (landscape) Page 2
Ch09 Alkynes (landscape) Page 2 When additional functional groups are present, the suffixes are combined. Terminal and Internal Alkynes The position of the triple bond can alter the reactivity of the alkyne. Compounds with triple bonds at the end of a molecule are called terminal alkynes. (Terminal C-H groups are called acetylenic hydrogens). If the triple bond is flanked by alkyl groups on both sides it is an internal alkyne
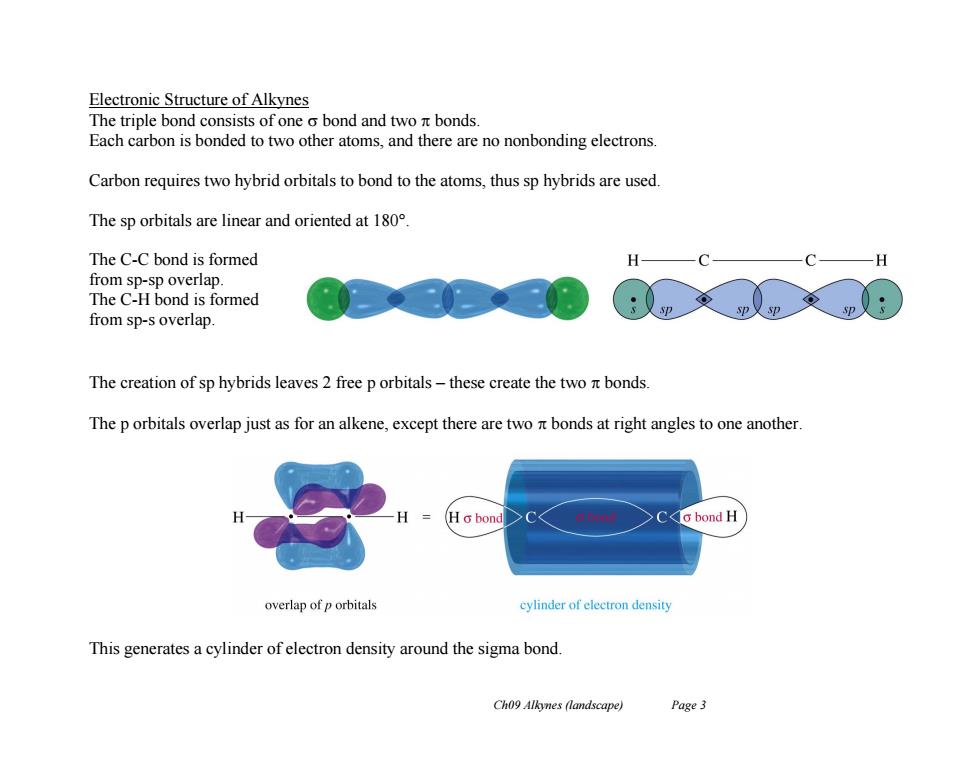
Electronic Structure of Alkynes The triple bond consists of one o bond and two nt bonds. Each carbon is bonded to two other atoms,and there are no nonbonding electrons Carbon requires two hybrid orbitals to bond to the atoms,thus sp hybrids are used. The sp orbitals are linear and oriented at 180 The C-C bond is formed H from sp-sp overlap. The C-H bond is formed from sp-s overlap. The creation of sp hybrids leaves 2 free p orbitals-these create the two nt bonds The p orbitals overlap just as for an alkene,except there are two nt bonds at right angles to one another. Hc bond o bond H overlap of p orbitals cylinder of eleetron density This generates a cylinder of electron density around the sigma bond. Ch09 Alkynes (landscape) Page 3
Ch09 Alkynes (landscape) Page 3 Electronic Structure of Alkynes The triple bond consists of one bond and two bonds. Each carbon is bonded to two other atoms, and there are no nonbonding electrons. Carbon requires two hybrid orbitals to bond to the atoms, thus sp hybrids are used. The sp orbitals are linear and oriented at 180°. The C-C bond is formed from sp-sp overlap. The C-H bond is formed from sp-s overlap. The creation of sp hybrids leaves 2 free p orbitals – these create the two bonds. The p orbitals overlap just as for an alkene, except there are two bonds at right angles to one another. This generates a cylinder of electron density around the sigma bond
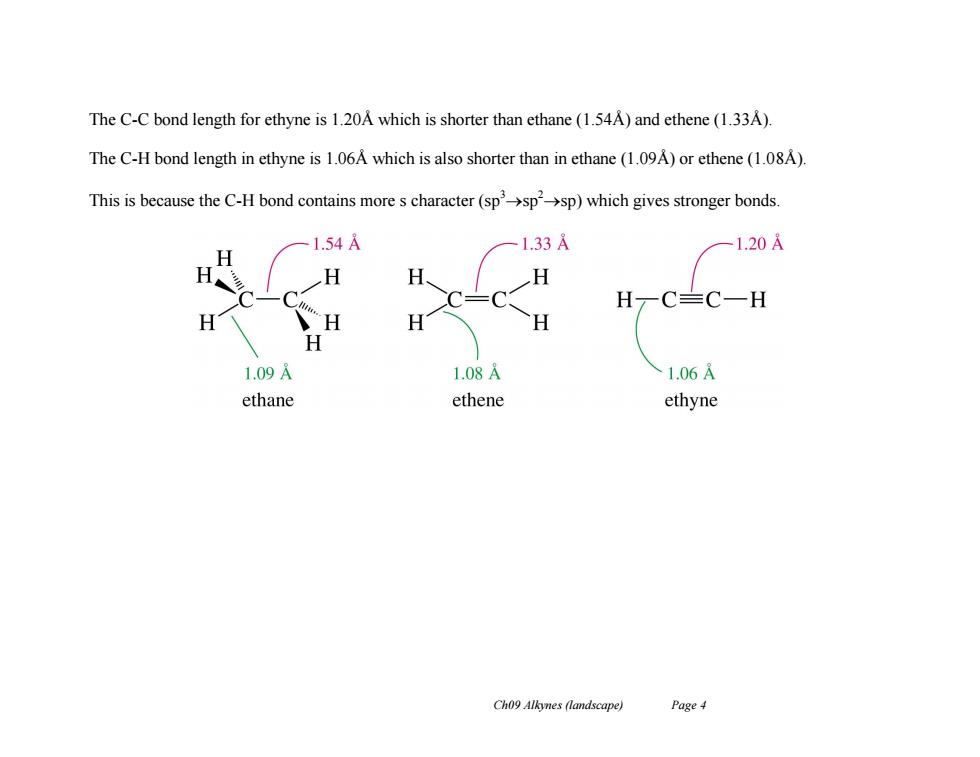
The C-C bond length for ethyne is 1.20A which is shorter than ethane(1.54A)and ethene(1.33A) The C-H bond length in ethyne is 1.06A which is also shorter than in ethane (1.09A)or ethene(1.08A). This is because the C-H bond contains more s character(sp'->sp->sp)which gives stronger bonds. 1.54A 1.33A 1.20A H H H C≡C一H H H H 1.09 1.08A 1.06A ethane ethene ethyne Ch09 Alkynes (landscape) Page4
Ch09 Alkynes (landscape) Page 4 The C-C bond length for ethyne is 1.20Å which is shorter than ethane (1.54Å) and ethene (1.33Å). The C-H bond length in ethyne is 1.06Å which is also shorter than in ethane (1.09Å) or ethene (1.08Å). This is because the C-H bond contains more s character (sp3sp 2sp) which gives stronger bonds
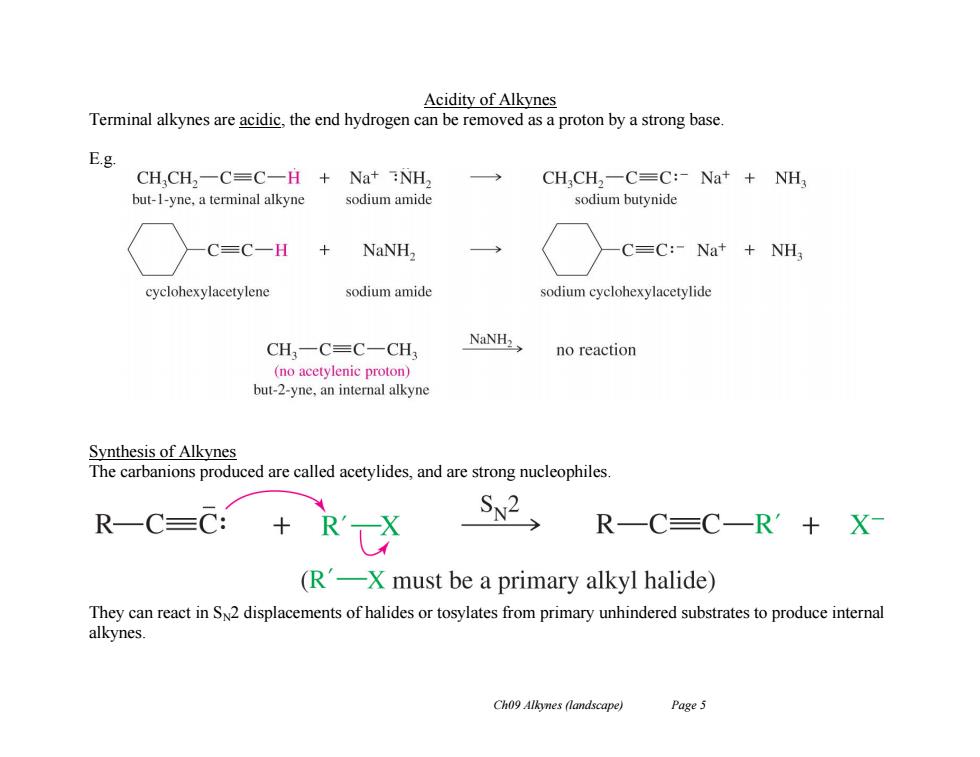
Acidity of Alkynes Terminal alkynes are acidic,the end hydrogen can be removed as a proton by a strong base. E.g CH,CH2一C=C一H+Na+NH, CHCH,-C=C:-Na+NH but-1-yne,a terminal alkyne sodium amide sodium butynide C=C一H NaNH2 C=C:-Na+NH, cyclohexylacetylene sodium amide sodium cyclohexylacetylide CH,一C=C-CH NaNH2> no reaction (no acetylenic proton) but-2-yne,an internal alkyne Synthesis of Alkynes The carbanions produced are called acetylides,and are strong nucleophiles. R-C=C: S2, R-C=C-R'十X (R-X must be a primary alkyl halide) They can react in SN2 displacements of halides or tosylates from primary unhindered substrates to produce internal alkynes. Ch09 Alkynes(landscape) Page 5
Ch09 Alkynes (landscape) Page 5 Acidity of Alkynes Terminal alkynes are acidic, the end hydrogen can be removed as a proton by a strong base. E.g. Synthesis of Alkynes The carbanions produced are called acetylides, and are strong nucleophiles. They can react in SN2 displacements of halides or tosylates from primary unhindered substrates to produce internal alkynes