
CNGNGE JOHN MCMURRY CHAPTER 26 Orbitals and Organic Chemistry: Pericyclic Reactions H IRD EDITION Organic Chemistry with Biological applications
CHAPTER 26 Orbitals and Organic Chemistry: Pericyclic Reactions
Pericyclic Reactions -What Are They? Pericyclic reaction All bonding changes occur simultaneously through a cyclic transition state The combination of steps is called a concerted process; no intermediates are involved Why this chapter? Understanding of pericyclic reactions emerged in the latter half of the twentieth century Some biological pathways proceed through pericyclic reactions
Pericyclic reaction • All bonding changes occur simultaneously through a cyclic transition state • The combination of steps is called a concerted process; no intermediates are involved Why this chapter? • Understanding of pericyclic reactions emerged in the latter half of the twentieth century • Some biological pathways proceed through pericyclic reactions Pericyclic Reactions – What Are They?
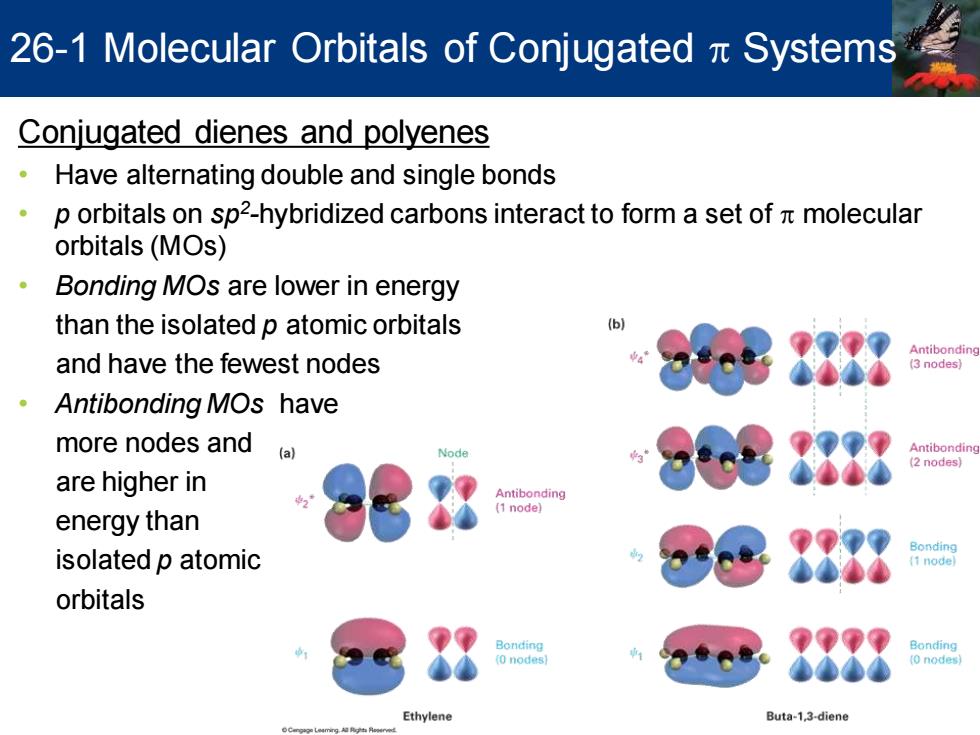
26-1 Molecular Orbitals of Conjugated Systems Conjugated dienes and polyenes Have alternating double and single bonds 。 p orbitals on sp2-hybridized carbons interact to form a set of molecular orbitals(MOs) 。 Bonding MOs are lower in energy than the isolated p atomic orbitals b Antibonding and have the fewest nodes 3 nodes ·Antibonding MOs have more nodes and (a) Antibonding 2 nodes) are higher in Antibonding (1 node) energy than isolated p atomic orbitals Bonding Bonding 0 nodes (0 nodes Ethylene Buta-1,3-diene
Conjugated dienes and polyenes • Have alternating double and single bonds • p orbitals on sp2 -hybridized carbons interact to form a set of p molecular orbitals (MOs) • Bonding MOs are lower in energy than the isolated p atomic orbitals and have the fewest nodes • Antibonding MOs have more nodes and are higher in energy than isolated p atomic orbitals 26-1 Molecular Orbitals of Conjugated p Systems
1,3,5-Hexatriene MO description for 1,3,5-Hexatriene -Three double bonds and six MOs -Only bonding orbitals,,w2,and wa,are filled in the ground state -On irradiation with ultraviolet light an electron is promoted from highest-energy filled orbital (y3)to the lowest-energy unfilled orbital (* Va and are each half-filled .This is the first (lowest energy)excited state
MO description for 1,3,5-Hexatriene •Three double bonds and six p MOs •Only bonding orbitals, ψ1 , ψ2 , and ψ3 , are filled in the ground state •On irradiation with ultraviolet light an electron is promoted from highest-energy filled orbital (ψ3 ) to the lowest-energy unfilled orbital (ψ4 *) •ψ3 and ψ4 * are each half-filled •This is the first (lowest energy) excited state 1,3,5-Hexatriene
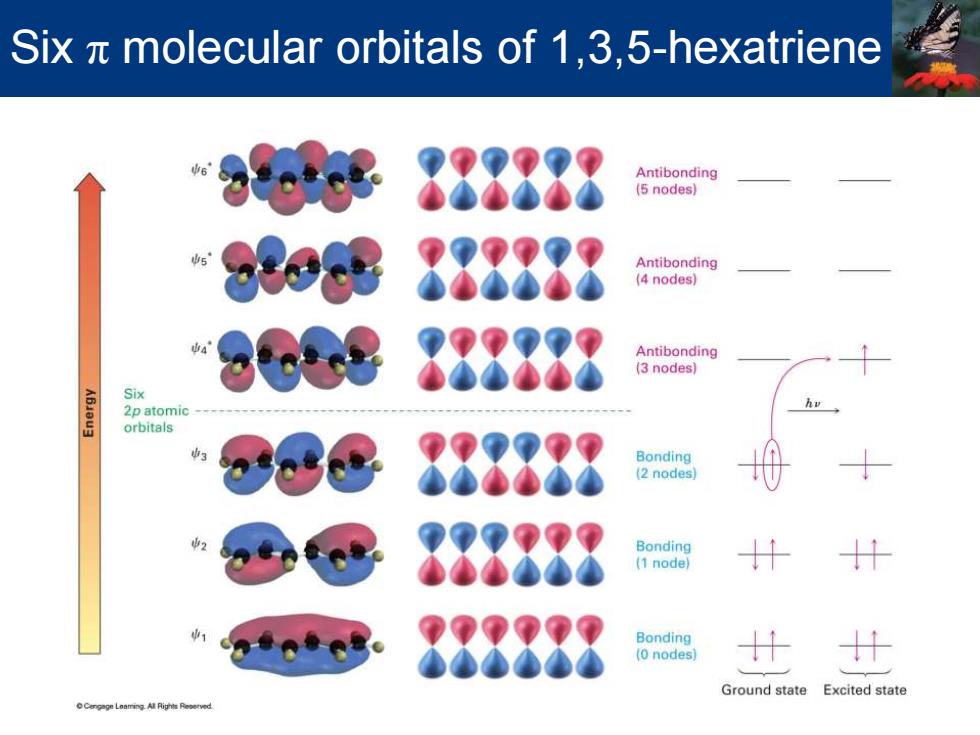
Six x molecular orbitals of 1,3,5-hexatriene 888888 Antibonding (5 nodes) 888888 Antibonding (4 nodes) 888888 Antibonding (3 nodes) Six 2p atomic orbitals Bonding (2 nodes) 888 Bonding 888888 Bonding (0 nodes 计 Ground state Excited state
Six π molecular orbitals of 1,3,5-hexatriene