Molecular Orbitals and Pericyclic Reactions Woodward-Hoffmann Rules for Pericyclic Reactions -Reaction can occur only if the symmetries of the reactant MOs are the same as the symmetries of the product MOs The lobes of reactant MOs must be of the correct algebraic sign for bonding to occur in the transition state leading to product If the symmetries of reactant and product orbitals correlate,the reaction is symmetry-allowed.Reactions conditions are relatively mild If the symmetries of the reactant and product orbitals do not correlate,the reaction is symmetry-disallowed and there are no low energy concerted paths Fukui's Simplification Frontier Orbitals -Consider only the frontier orbitals-the highest occupied molecular orbital (HOMO)and the lowest unoccupied molecular orbital (LUMO)
Woodward-Hoffmann Rules for Pericyclic Reactions •Reaction can occur only if the symmetries of the reactant MOs are the same as the symmetries of the product MOs • The lobes of reactant MOs must be of the correct algebraic sign for bonding to occur in the transition state leading to product • If the symmetries of reactant and product orbitals correlate, the reaction is symmetry-allowed. Reactions conditions are relatively mild • If the symmetries of the reactant and product orbitals do not correlate, the reaction is symmetry-disallowed and there are no low energy concerted paths Fukui’s Simplification – Frontier Orbitals •Consider only the frontier orbitals – the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) Molecular Orbitals and Pericyclic Reactions
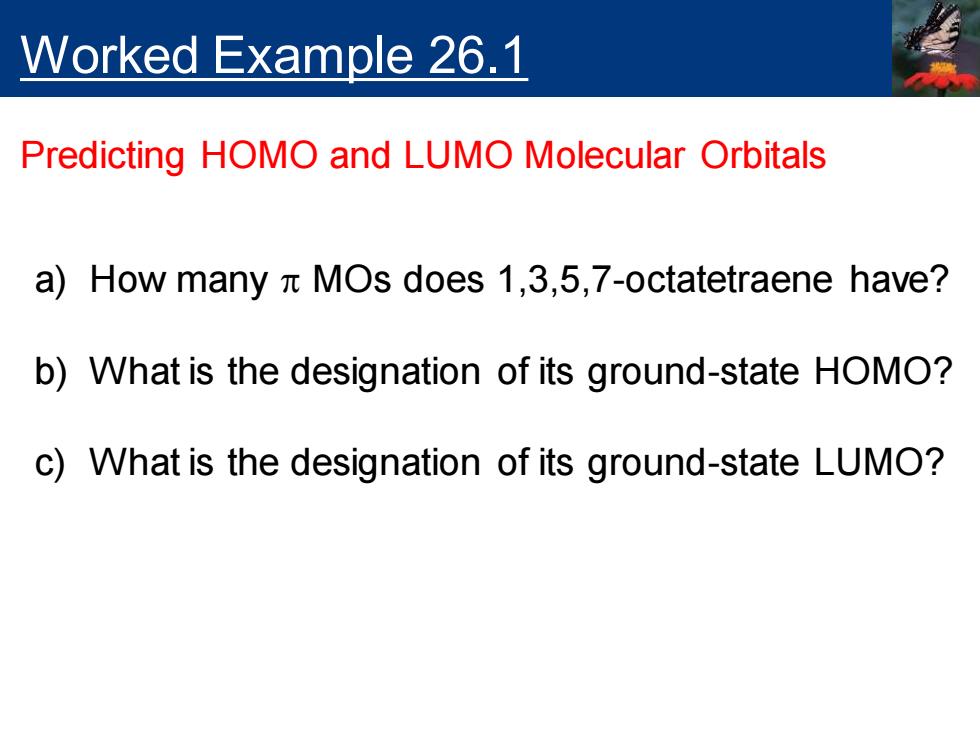
Worked Example 26.1 Predicting HOMO and LUMO Molecular Orbitals a)How many n MOs does 1,3,5,7-octatetraene have? b)What is the designation of its ground-state HOMO? c)What is the designation of its ground-state LUMO?
Worked Example 26.1 Predicting HOMO and LUMO Molecular Orbitals a) How many p MOs does 1,3,5,7-octatetraene have? b) What is the designation of its ground-state HOMO? c) What is the designation of its ground-state LUMO?
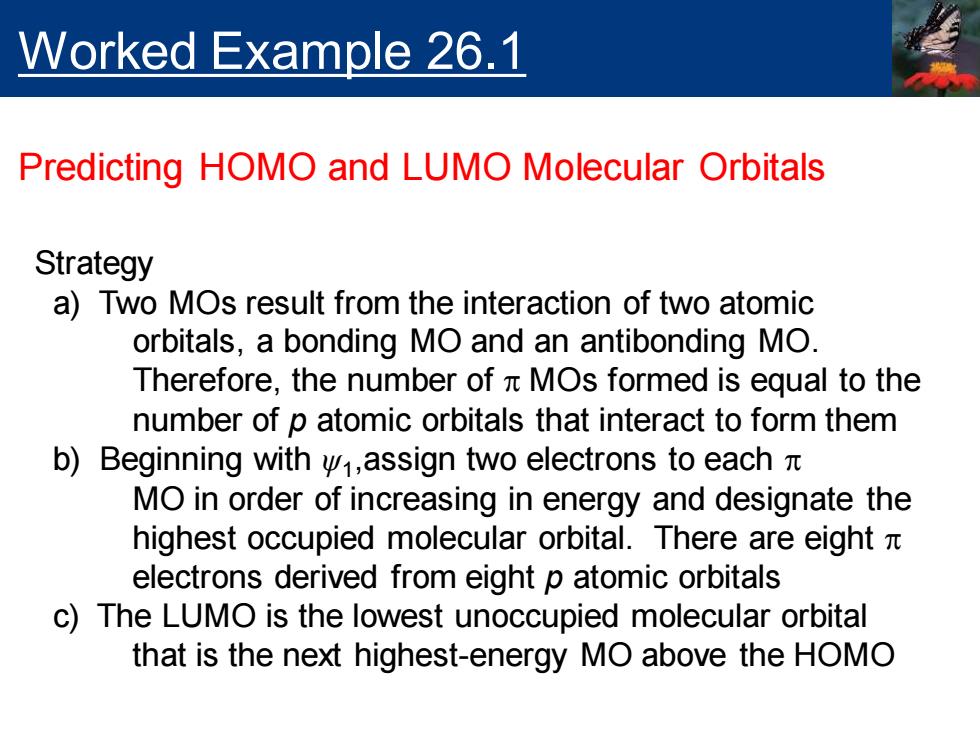
Worked Example 26.1 Predicting HOMO and LUMO Molecular Orbitals Strategy a)Two MOs result from the interaction of two atomic orbitals,a bonding MO and an antibonding MO. Therefore,the number of n MOs formed is equal to the number of p atomic orbitals that interact to form them b)Beginning with v,assign two electrons to each MO in order of increasing in energy and designate the highest occupied molecular orbital.There are eight x electrons derived from eight p atomic orbitals c)The LUMO is the lowest unoccupied molecular orbital that is the next highest-energy MO above the HOMO
Worked Example 26.1 Predicting HOMO and LUMO Molecular Orbitals Strategy a) Two MOs result from the interaction of two atomic orbitals, a bonding MO and an antibonding MO. Therefore, the number of p MOs formed is equal to the number of p atomic orbitals that interact to form them b) Beginning with ψ1 ,assign two electrons to each p MO in order of increasing in energy and designate the highest occupied molecular orbital. There are eight p electrons derived from eight p atomic orbitals c) The LUMO is the lowest unoccupied molecular orbital that is the next highest-energy MO above the HOMO
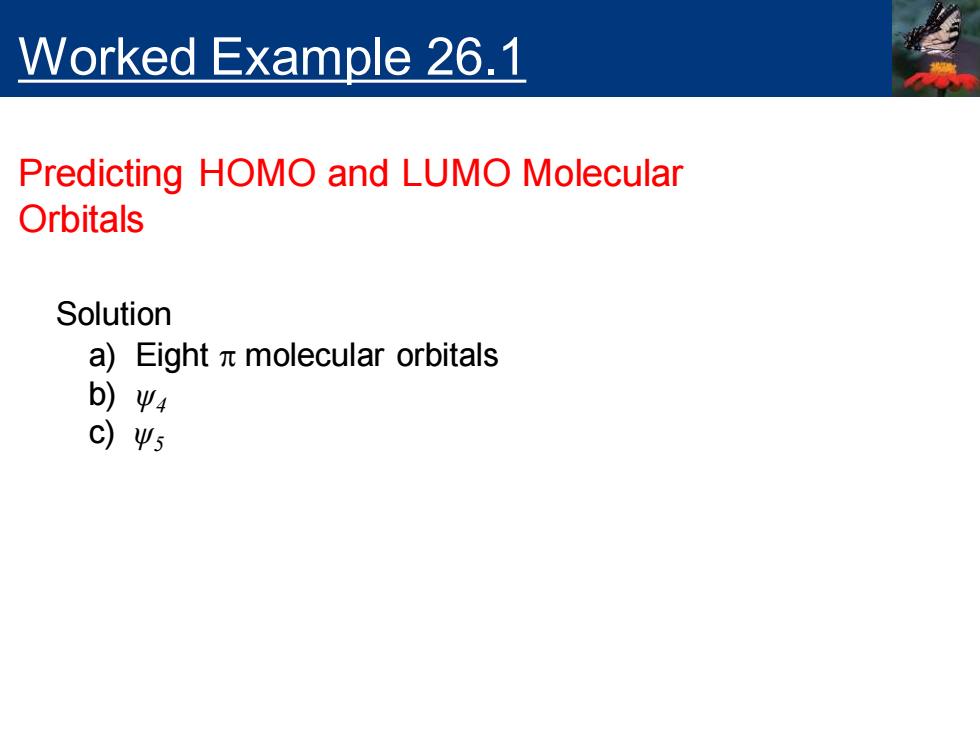
Worked Example 26.1 Predicting HOMO and LUMO Molecular Orbitals Solution a)Eight n molecular orbitals b)Ψ4 c)Ψ5
Worked Example 26.1 Predicting HOMO and LUMO Molecular Orbitals Solution a) Eight p molecular orbitals b) ψ4 c) ψ5
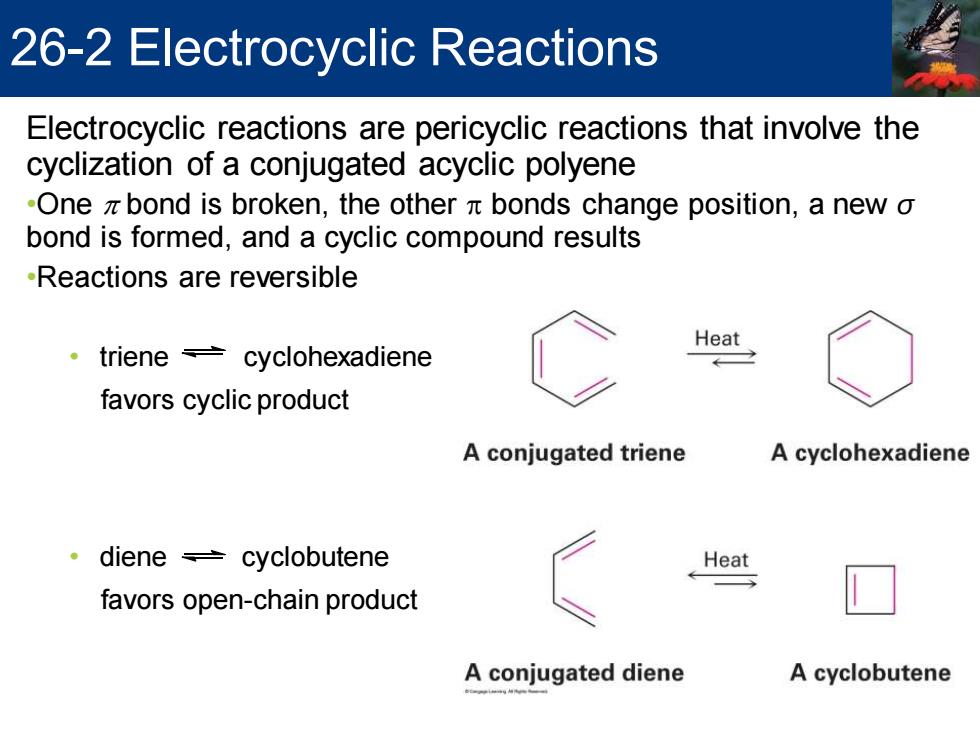
26-2 Electrocyclic Reactions Electrocyclic reactions are pericyclic reactions that involve the cyclization of a conjugated acyclic polyene -One z bond is broken,the other x bonds change position,a new o bond is formed,and a cyclic compound results -Reactions are reversible Heat triene cyclohexadiene favors cyclic product A conjugated triene A cyclohexadiene ·diene≠cyclobutene Heat favors open-chain product A conjugated diene A cyclobutene
Electrocyclic reactions are pericyclic reactions that involve the cyclization of a conjugated acyclic polyene •One p bond is broken, the other p bonds change position, a new σ bond is formed, and a cyclic compound results •Reactions are reversible • triene cyclohexadiene favors cyclic product • diene cyclobutene favors open-chain product 26-2 Electrocyclic Reactions