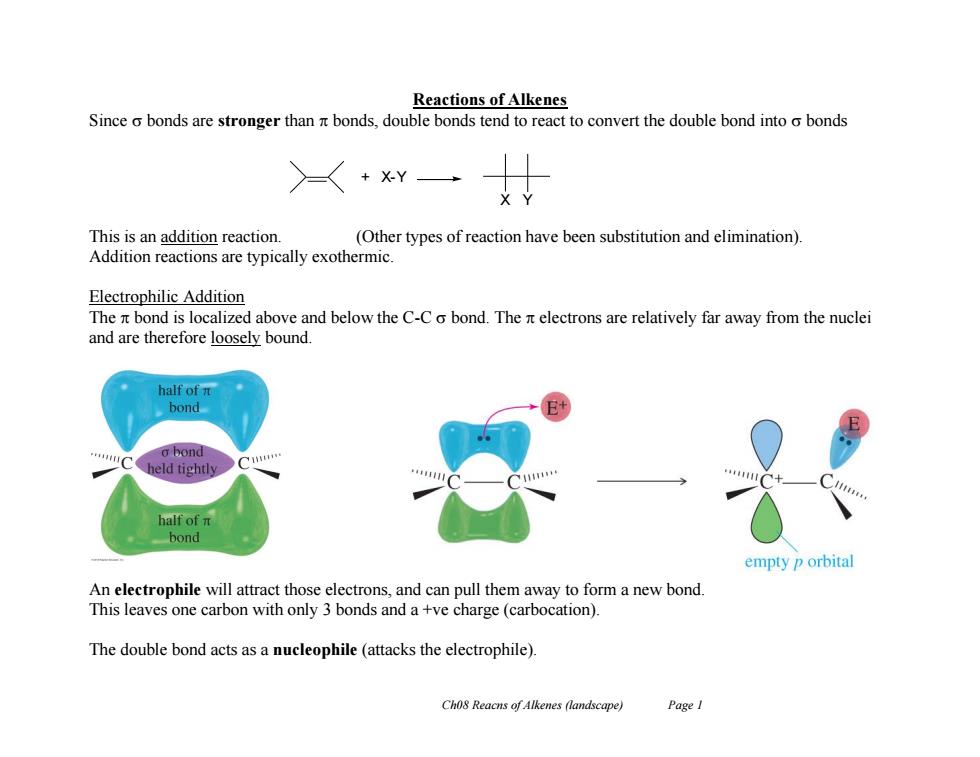
Reactions of Alkenes Since o bonds are stronger than nt bonds,double bonds tend to react to convert the double bond into o bonds >+xY This is an addition reaction (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The it bond is localized above and below the C-C o bond.The it electrons are relatively far away from the nuclei and are therefore loosely bound. half of n bond o bond held tightly half of n bond empty p orbital An electrophile will attract those electrons,and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile(attacks the electrophile). Cho8 Reacns of Alkenes (landscape) Page I
Ch08 Reacns of Alkenes (landscape) Page 1 Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The bond is localized above and below the C-C bond. The electrons are relatively far away from the nuclei and are therefore loosely bound. An electrophile will attract those electrons, and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile (attacks the electrophile). + X-Y X Y
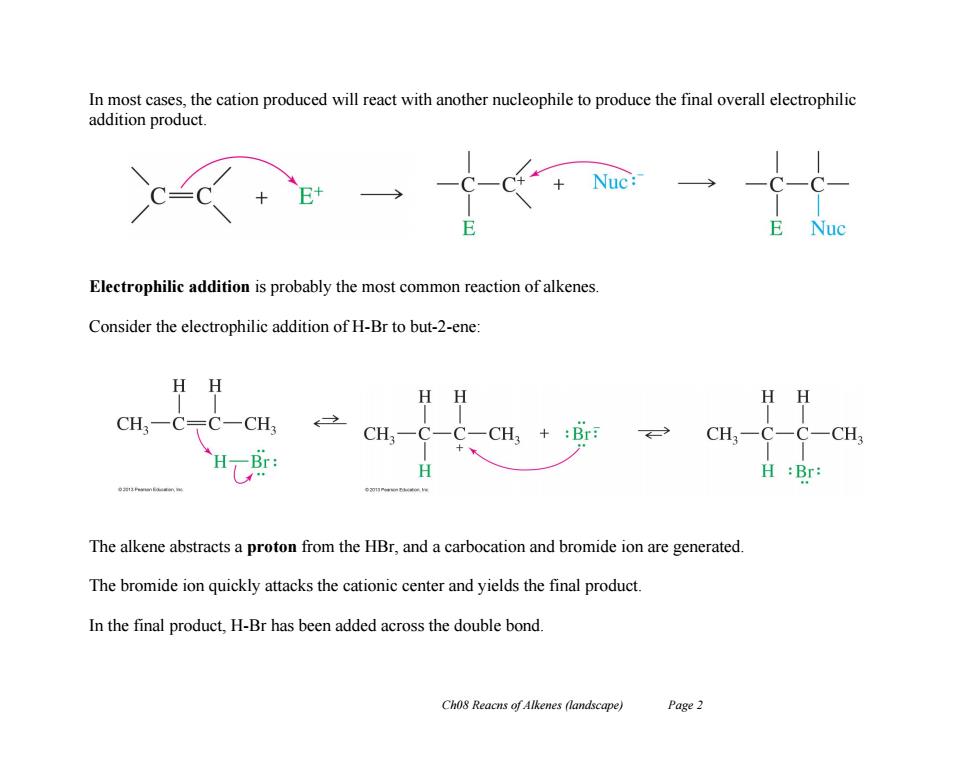
In most cases,the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: H HH CH. CH. CH, +:Br CH3一C-C-CH3 Br H:Br: The alkene abstracts a proton from the HBr,and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product,H-Br has been added across the double bond. Cho8 Reacns of Alkenes (landscape) Page 2
Ch08 Reacns of Alkenes (landscape) Page 2 In most cases, the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: The alkene abstracts a proton from the HBr, and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product, H-Br has been added across the double bond
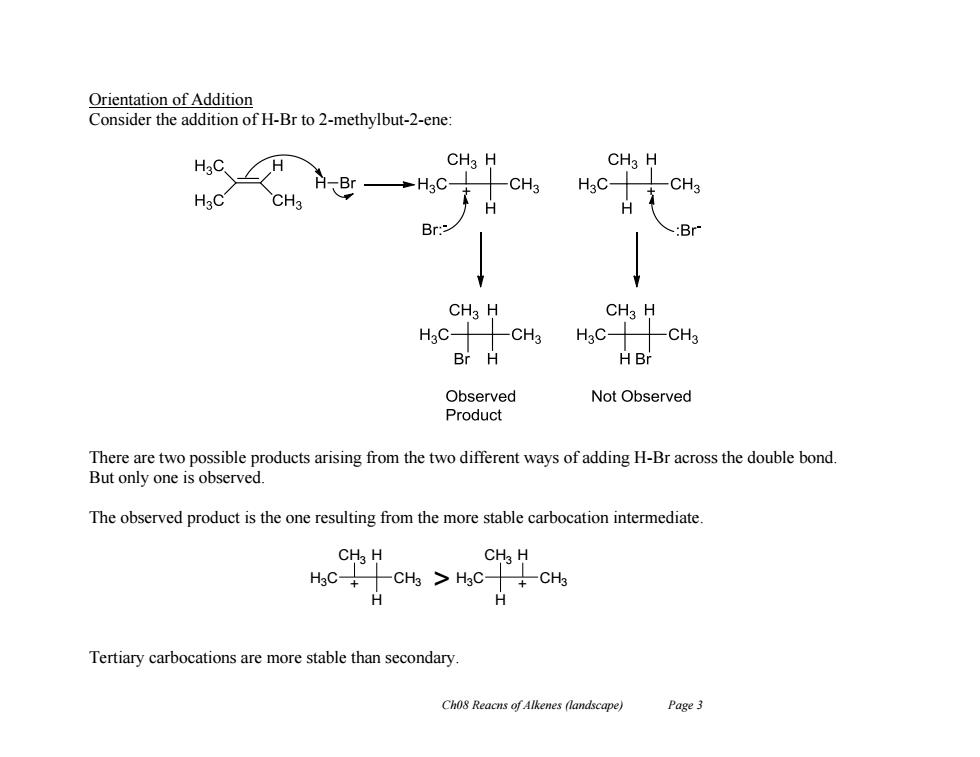
Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: CHa H CHa H -CH3 H3C- -CH3 CH3 HBr→HC H Br: :Br CH3 H CH3 H H3C- CH3 HgC-CH3 Br H HBr Observed Not Observed Product There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. CH H CH H HaCCHs HgC-+CH3 H H Tertiary carbocations are more stable than secondary. Cho8 Reacns of Alkenes (landscape) Page 3
Ch08 Reacns of Alkenes (landscape) Page 3 Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. Tertiary carbocations are more stable than secondary. H3C CH3 H CH3 H + CH3 CH3 H H3C H > +
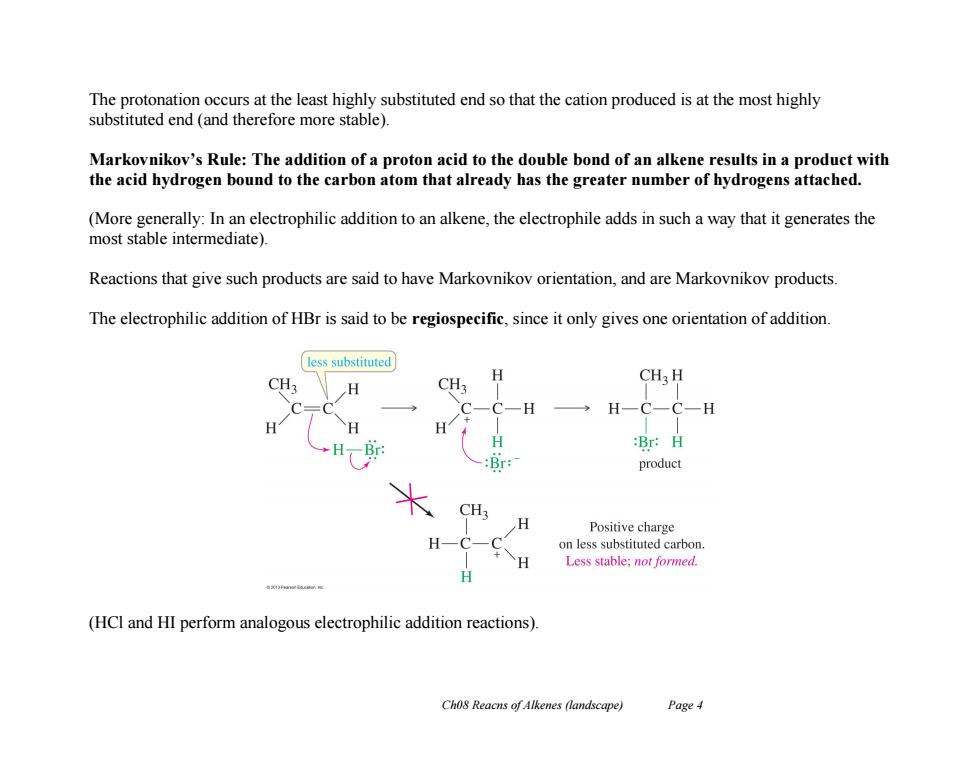
The protonation occurs at the least highly substituted end so that the cation produced is at the most highly substituted end (and therefore more stable). Markovnikov's Rule:The addition of a proton acid to the double bond of an alkene results in a product with the acid hydrogen bound to the carbon atom that already has the greater number of hydrogens attached. (More generally:In an electrophilic addition to an alkene,the electrophile adds in such a way that it generates the most stable intermediate). Reactions that give such products are said to have Markovnikov orientation,and are Markovnikov products. The electrophilic addition of HBr is said to be regiospecific,since it only gives one orientation of addition. less substituted CH3 H H CH H -Br :Br:H :Br: product CH H Positive charge H C-H on less substituted carbon. Less stable;not formed. H (HCI and HI perform analogous electrophilic addition reactions). Cho8 Reacns of Alkenes (landscape) Page 4
Ch08 Reacns of Alkenes (landscape) Page 4 The protonation occurs at the least highly substituted end so that the cation produced is at the most highly substituted end (and therefore more stable). Markovnikov’s Rule: The addition of a proton acid to the double bond of an alkene results in a product with the acid hydrogen bound to the carbon atom that already has the greater number of hydrogens attached. (More generally: In an electrophilic addition to an alkene, the electrophile adds in such a way that it generates the most stable intermediate). Reactions that give such products are said to have Markovnikov orientation, and are Markovnikov products. The electrophilic addition of HBr is said to be regiospecific, since it only gives one orientation of addition. (HCl and HI perform analogous electrophilic addition reactions)

Free Radical addition to Alkenes It is possible to obtain anti-Markovnikov products when HBr is added to alkenes in the presence of free radical initiators. The free radical initiators change the mechanism of addition from electrophilic addition to free radical addition. This change of mechanism gives rise to the opposite regiochemistry Initiation: 6-R R-+·-R R-0+H- R-O一H+:Br The oxygen-oxygen bond is weak,and is easily homolytically cleaved to generate two alkoxy radicals,which in turn abstract hydrogen to generate bromine radicals. Cho8 Reacns of Alkenes (landscape) Page 5
Ch08 Reacns of Alkenes (landscape) Page 5 Free Radical addition to Alkenes It is possible to obtain anti-Markovnikov products when HBr is added to alkenes in the presence of free radical initiators. The free radical initiators change the mechanism of addition from electrophilic addition to free radical addition. This change of mechanism gives rise to the opposite regiochemistry. Initiation: The oxygen-oxygen bond is weak, and is easily homolytically cleaved to generate two alkoxy radicals, which in turn abstract hydrogen to generate bromine radicals